USGS Professional Paper 144 Butler & Burbank
MINERALOGY
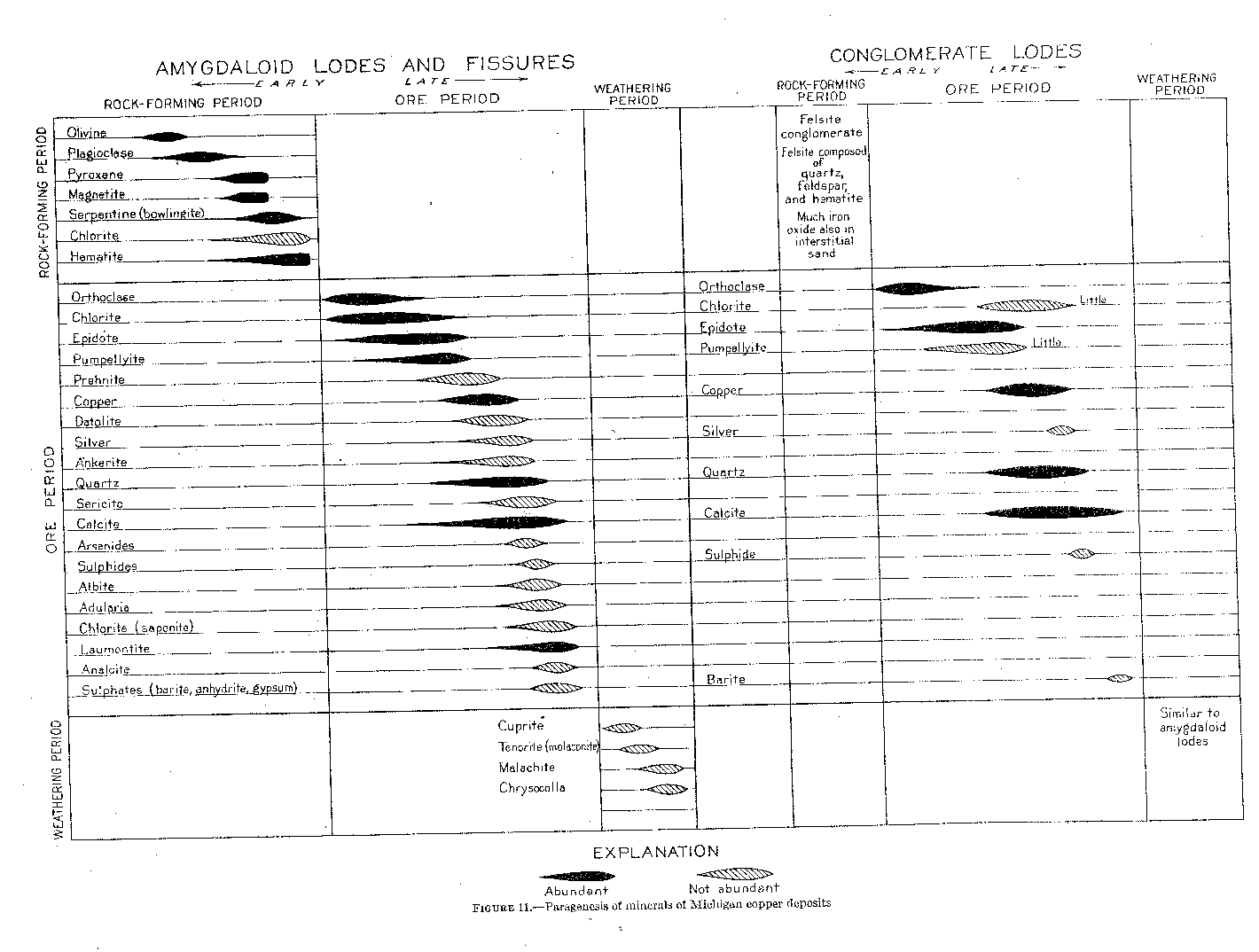
The following discussion of the mineralogy based largely on the notes of Charles Palache and Alfred Wandke. Doctor Palache made a study of the general mineralogy of the district, and Doctor Wandke did much of the microscopic work on rocks and minerals. In addition Dr. T. M. Broderick made a special study of the arsenides. The work of these men was carried on for the Calumet & Hecla Consolidated Copper Co. and has been supplemented by observations made throughout the investigation.The minerals of the district readily fall into three main groups, based upon their periods of formation those of the rock-forming period, those of the ore-forming period, and those of the period of weathering. (See fig. 11.)

Figure 11 - Paragenesis of minerals of Michigan copper deposits
ROCK-FORMING PERIOD
The cooling of the basaltic lava flows resulted in the crystallization of olivine, plagioclase, pyroxene, and magnetite or ilmenite, together with the formation of more or less glass. Gas bubbles in the viscous lavas left open cavities or vesicles. Interstices in the ophites may also have been gas filled and later left open or filled with glass.
As the rock cooled and crystallized, it was permeated with gaseous emanations, which may have been the chief agents in producing the first alterations of the minerals or glass already formed. These changes were principally the breaking down of glass, with setting free of dissolved minerals - hematite, pyroxene, and feldspar (in spherulitic form); the serpentinization of olivine, with or without setting free of iron oxide, mainly hematite; probably the formation of chlorite from glass of interstitial spaces or migration to fill such areas if open; and the oxidation of ferrous iron in the glass, magnetite, and silicates to hematite. It was in this period that the red tops of the lavas were formed.
During the corresponding period in the formation of felsites, quartz, feldspar, and hematite were the principal minerals to be formed. Some of the felsites were later broken up and deposited to form the conglomerates with little mineral change.
ORE-FORMING PERIOD
The main ore-forming period occurred after the rocks had been tilted to essentially their present position and broken by many fractures. They were then permeated by hot, chemically active solutions, which tended to rearrange the constituents of the rocks into new mineral combinations and also to introduce some additional constituents.
Within this period there was a broad general sequence of mineral formation; but this sequence was subject to many variations and is likely to be obscured by its own complexity. The early part of the period was characterized by the formation of the anhydrous or less hydrous minerals, such as feldspar, chlorite, and epidote; the later part by the formation of the more hydrous minerals, such as laumontite, analcite, and saponite. (See fig. 11.) Copper was formed mainly in the intermediate part of the period.
Many of the minerals were formed along a path leading away from a source of solutions, as outward from a fissure or channelway, and minerals of one type would be forming at the advancing front of the replacement wave while minerals of another type were replacing these earlier minerals nearer the source at the same time and perhaps only a few inches away. Thus the front of the replacement wave in amygdaloid is marked by the destruction of hematite and the formation of epidote and pumpellyite, but these same minerals nearer the source of the solutions were being replaced by copper. In the iron-rich boulders in the Calumet & Hecla conglomerate the rock was chloritized at the front of the replacement wave and replaced by copper a little nearer the source, both processes evidently having been in progress at the same time and, in places, but a fraction of an inch apart.
The complexity of this period is illustrated by the relations of minerals in the Allouez shatter zone, where movement was in progress during mineralization and there was repeated opening of fissures and healing with other minerals. (See pls. 64, 65.) The general sequence of events in this zone is believed to have been as follows:1. Fissuring occurred, and quartz and epidote were formed.
2. During the silicification of the fissure walls a little of the hematite, which antedated the fissuring, was removed.
3. As epidote developed, hematite decreased in amount, owing in part to the recombination of hematite into epidote.
4. As silicification proceeded, a little of the pulverulent ferric oxide may have recrystallized into the specular black variety.
5. A little pumpellyite was formed.
6. The early quartz-epidote mixture was shattered, and quartz, epidote, calcite, and prehnite were deposited in the fractures.
7. Prehnite partly replaced epidote, quartz, and calcite, and copper was deposited.
8. The minerals already formed were again shattered, and more calcite and quartz were deposited, together with copper.
9. Shattering was renewed, and quartz and calcite, with some laumontite, entered.
The
minerals of this period both filled open cavities and replaced minerals of the
rock-forming period and the earlier part of the ore-forming period. The
replacement generally occurred volume for volume, but in places the altering
solutions removed more than they deposited and formed cavities near the main channels,
which may have become places of deposit for later minerals. The veins were
formed mainly by replacement, though there was doubtless some filling of open
spaces:
PERIOD OF WEATHERING
The glaciation of the region has removed most of the products of preglacial weathering. It is only in areas protected from glacial action or areas of shattering where weathering was probably unusually deep or in association with minerals that were readily susceptible to oxidation that any notable amount of weathered material is present. In weathering native copper has generally, as is usual in other regions, been altered first to oxide and later to carbonate or silicate.
MINERALS OF THE KEWEENAWAN COPPER-BEARING
ROCKSIn the following alphabetic list are included the principal known minerals of the district, and following that is a short description of the occurrence of each. In the description the minerals are grouped according to Dana's system.
NATIVE METALS
Silver (Ag) occurs in varying amounts in all the lodes and fissures. Among the fissures the Cliff has probably been most productive of silver and among the lodes the Pewabic. Silver occurs in the sulphide and arsenide fissures as well as in the native copper fissures. Some of it was formed at the same time as the associated copper, though in its most conspicuous occurrences in vugs it was apparently formed slightly later than most of the copper. (See pl. 67.)
Native copper (Cu) occurs in amygdaloids, conglomerates, and fissures throughout the district. It forms masses ranging from those of microscopic size to some weighing 600 tons. Commonly it is without definite crystal outline, but in vugs it occurs in crystals and crystal aggregates, usually with rather imperfect crystal form. This crystallized copper has apparently been deposited in open spaces, without replacing other minerals. Some crystallized copper has formed in fault gouge. In the main the metal has been formed by replacement of the earlier minerals of the ore period, such as chlorite, epidote, and zoisite, as well as of the rock-forming minerals. It is very commonly intergrown with prehnite and datolite and less commonly with quartz and calcite. Most of the copper is earlier than the zeolites laumontite and analcite and earlier than saponite, adularia, barite, anhydrite, and gypsum, though a little copper has been deposited as late as any of these minerals. Where copper is associated with chalcocite in fissures either mineral may be the earlier, or the two may be contemporaneous. In the lodes the sulphide-bearing fissures are at least in part later than the copper of the lodes. The lode rock adjacent to copper is characteristically bleached by the removal and alteration of hematite, as is discussed in the section on ore deposits (p. 133).
SULPHIDES
Galena (PbS) is rare, but minute crystals of it have been noted at the Mendota diamond-drill hole No. 40 with chalcopyrite and at South Cliff with pyrite and sphalerite.
Chalcocite (Cu
2S), though formed before copper in some places, is one of the later minerals of the ore-forming period. It is of widespread occurrence in fissures cutting rocks of all kinds. It is especially conspicuous in fissures in the Baltic lode, where it occurs with ankerite, and it is also present locally in the lode itself. It is rather common in fissures in the Isle Royale lode, where it occurs with ankerite and a little specularite and where it is later than the specularite. The rock adjacent to the veins in this lode is strongly bleached. Finely divided chalcocite darkens the small calcite veins that occur in the Allouez conglomerate wherever it has been opened, and the mineral occurs similarly in several other conglomerates. In the Calumet & Hecla conglomerate it is most abundant at the Centennial shafts, north of the main ore shoot. It is present in veins and vugs at Mount Bohemia. Calcite-chalcocite veins are plentiful in the White Pine and Carp Lake mines, and chalcocite is disseminated in part of the sandstone. In short, chalcocite is widespread but nowhere abundant, though the Baltic lodes contain veins of nearly solid chalcocite several inches wide. (See pls. 68, 69.) Sphalerite (ZnS) is rare but is reported from a fissure at South Cliff with pyrite and galena.Bornite (Cu
5FeS4) in small amount accompanies the chalcocite in some of the sulphide fissures. The fissures in the Baltic lode contain bornite most abundantly, but it is present also in fissures in the Isle Royale lode. It is a common mineral in the veins and vugs in the gabbro and gabbro aplite of Mount Bohemia.Chalcopyrite (CuFeS
2) is of rather rare occurrence. It is present in the veins of Mount Bohemia and was seen in drill cores near a felsite intrusive at Mendota. It occurs sparingly in veins with chalcocite in the Isle Royale and Baltic lodes and was observed in veins in quartz porphyry in the Onondaga drill cores. It is visible in some of the pegmatitic lenses in the thicker flows and is present as microscopic crystals in some of the freshest normal trap. It occurs also in the Wolverine sandstone in the Ahmeek mine.Pyrite (FeS
2) is reported in a small fissure with sphalerite and galena at South Cliff. Pyrite is notable for its practical absence.ARSENIDES
Arsenic, in the form of copper arsenides (pl. 71), occurs in Michigan associated with the copper in deposits of all types - amygdaloid and conglomerate lodes and fissures - but is most abundant in certain of the fissure deposits, and in the lodes it is especially localized near the arsenide fissures. The lode copper of the Baltic and Isle Royale lodes, however, is arsenical throughout and distinctly lighter in color than the arsenic-free copper. On etched surfaces the "arsenical" copper approaches in appearance some of the arsenic compounds.
Compounds of copper and arsenic . - As they occur in Michigan, the arsenides form mixtures so intimate and complex that the early investigators67 mistook different combinations of minerals for new species. Examination in polished section shows at least seven recognizable varieties, not including three that contain nickel and cobalt. It has not been possible to separate these varieties and determine their chemical composition by analyses, and the composition assigned to them is that given in the textbooks. It is entirely possible that some of them, especially those high in copper and low in arsenic; may be solid solutions or alloys and not definite minerals. Domeykite (Cu3As) is highest in arsenic, and it was the earliest to form, being preceded only by magnetite and the nickel-cobalt arsenides. Domeykite as veined and replaced by algodonite, whose formula has been given as Cu6As. Etching polished surfaces of Algodonnite shows that it is not a simple substance but an intergrowth of two minerals, which are here designated α and β algodonite. Algodonite is in turn succeeded and in part replaced by substances still higher in copper; several of these are now recognized, though all were formerly called whitneyite and. assigned the formula Cu9As. These are here designated α, β, γ, and δ whitneyite. They may well be solid solutions or alloys rather than definite compounds. Etching tests show that these substances are veined by native copper. Thus there is a definite order of deposition in the series. This order is invariably domeykite, algodonite, whitneyite, and native copper. Each mineral partly replaced its immediate predecessor; domeykite, so far as observed, has not been replaced by whitneyite or native copper, nor algodonite by native copper. Each arsenide was followed by the one next higher in copper content, until finally native copper was precipitated. The order of deposition of the individual members of the general algodonite and whitneyite group can not be so definitely stated. Even this, however, seems to be consistent with the general rule of elimination of arsenic. For example, where whitneyite was replaced or followed by copper, the whitneyite is of the γ or δ variety, but the whitneyite that replaced algodonite is in most places the lighter, more arsenical α or β whitneyite. In some specimens two members of the whitneyite group seem to grade into each other, instead of having sharply defined contacts. Every specimen of algodonite so far examined is an intimate intergrowth of the two constituents α and β algodonite. Where α predominates over β the color of the mixture is tin white, but with a predominance of β the specimen has a distinct pinkish tone, as if β had a higher copper content. The algodonite that has replaced domeykite generally has a predominance of the α variety, but where algodonite is replaced by whitneyite the β variety is predominant.Although the physical and chemical properties as described serve in general to determine roughly the arsenides present in a given specimen, examination in polished section makes it passable to differentiate varieties that are not distinguishable in the hand specimen and to determine their paragenesis. Below is a list of the copper. arsenides arranged in the order of their known or inferred copper content, together with their diagnostic properties in polished section.
Diagnostic
properties of copper arsenides
| Name | Composition | Color of polished surface in oblique light | Results of etching polished surface | Remarks | ||
| HNO3 | HCl | NH4OH | ||||
| Domeykite | Cu3As | white | Effervescence blackens. | Color unchanged, develops Δ cleavage patterns. | Turns yellow-brown. | After rubbing on plate with fine abrasive is dull gray compared with algodonite under similar conditions. |
| Algodonite | Cu6As | |||||
| α | Creamy white | Effervescence deep purple. | Brings out faint difference between α and β | Blackens | After etching with HNO3, polish gently and α is smooth β is white | |
| β | Pinkish creamy white. | Effervescence dark brown | ||||
| Whitneyite | Cu9As | |||||
| α | Deep creamy white | Effervescence | Faint brown rubs off | Little effect | In general the series shows colors grading from algodonite to that of pure copper. γ is decidedly pinker than others. δ is decidedly lighter than copper. All characterized by immediate browning with HCl. | |
| β | Deep cream | do | Deep brown rubs off | do | ||
| γ | pink (like breithauptite?) | Effervescence | Deep chocolate-brown rubs off. | do | ||
| δ | Copper-cream | Effervescence | Deep brown rubs off | do | ||
| Copper | Cu | Pink (brownish pink with &gamma whitneyite) | No color change | Fine solution pits, dull but no color change. | do | The color and lack of browning by HCl used to distinguish from whitneyite group. |
The several groups of arsenides are not difficult to recognize in hand specimens. The colors range from the white of domeykite and algodonite through the pale copper-reds of the whitneyite group to the copper-red of pure copper. One per cent of arsenic in arsenical copper causes a perceptible lightening of the color. The malleability of the minerals also varies. Domeykite is decidedly brittle, algodonite is much less so, and the whitneyite group is decidedly malleable but less so than pure copper. (See pl. 70.)
Heating in glass tubes drives off the arsenic easily from domeykite and algodonite, but with extreme difficulty from whitneyite, the lower arsenic compound.
The alterations of the arsenides are characteristic. Domeykite rapidly tarnishes to a chalcopyrite-yellow. Some has weathered to cuprite. The bright-green coloration that has developed along the arsenide fissures underground since they were opened by mining is probably due to the formation of an arsenate and seems to be chiefly connected with algodonite. Whitneyite tarnishes brown rather quickly.
Origin. --- It is pointed out in the section on ore deposits (p. 132) that in general, although there are some notable exceptions, the higher the lode is in the series the lower its arsenic content. This is also true of the occurrence of sulphides of copper, and what is said of the distribution of arsenic may be said of the distribution of sulphur. The explanation of this relation, which seems to be consistent with the chemical theory favored by the writers, is that the solutions which traversed the lower beds had not been in intimate contact with the oxidizing environment of the lodes for as long a time as those which deposited copper in the higher beds, and for that reason their arsenic and sulphur failed to become completely oxidized into a more soluble state. There are three possible reasons why those solutions should have had less opportunity for oxidation: (1) The rocks toward the base of the series are more fissured than those higher up, hence solutions could find in the fissures a more permeable pathway than the oxidized amygdaloids. The arsenides and sulphides occur by far the most abundantly in fissures. (2) The occurrence of several intrusive bodies toward the base of the series may mean that a magmatic source for the copper lies much nearer the present surface in this part of the series than elsewhere. Thus the failure of complete oxidation of the solutions may be due to the fact that in that general area they were too close to the magmatic source. (3). Similarly, if the shattered zone associated with the Keweenaw fault is regarded as the main trunk channel for solutions emanating from a deep magmatic source, such solutions had not been in contact with an oxidizing environment sufficiently long when they traversed the rocks adjacent to that trunk channel.
The presence of sulphides in the Nonesuch lode near the Porcupine Mountains does not help in deciding which of these possible causes had the greatest effect, for major faulting, fissuring, and intrusive rocks are all present near by.
Another possibility which merits consideration is that the deposition of arsenides and sulphides in the fissures occurred subsequent to the main period of mineralization and was due to a change in the character of the solutions, the later ones being higher in sulphur and arsenic.
It is certain that in his investigations of these copper arsenides Koenig68 had very complex mixtures to deal with. His mohawkite and keweenawite and other new species were undoubtedly such mixtures. Modern metallographic methods were not available at the time he did his work.
It is to be hoped that the problems of these arsenides, the solutions of which have just been started in this work, will be further studied by others. The present writers have specialized on the material found in the Michigan copper district. It will be interesting to learn what specimens from other districts reveal. It would seem that chemical analyses and metallographic work upon synthetic compounds of copper and arsenic, if cooling curves are taken and the constitution diagram of those metals is kept in mind, would throw some light on the nature of the minerals.
Cobalt and nickel minerals have been recognized in a fissure in the Seneca mine, on the third level, 2,100 feet north of No. 2 shaft. The minerals present in the other arsenide fissures crossing the Kearsarge are present in this fissure - that is, domeykite, algodonite, and whitneyite. The microscope reveals in addition at least three other arsenides, one a white mineral, another a pinkish white, and the third a brownish pink, like niccolite. Chemical tests by Mr. Hillenbrand at the Calumet & Hecla smelter show abundant nickel. Borax bead tests show cobalt. The late George Heath, of the Calumet & Hecla smelter, stated that in the arsenides of the Ahmeek fissures there is but a trace of nickel. Therefore the nickel and cobalt are probably accounted for by the new minerals. The sequence in age seems to be, first, the white mineral, next the pinkish white, then the domeykite, followed by the other copper arsenides in their usual order. The brownish-pink mineral is present in very minor amounts in the sections seen, and its relations were not determined. The magnetite was formed early in the nickel-cobalt stage.
The tests on the white and pinkish-white minerals so far have not corresponded to those of any described mineral. The pinkish-white mineral shows the following properties in polished section: Hardness, that of magnetite; effect of HNO3, effervesces, fumes slightly brown, no coating or etching; of HCl, negative; of HgCl2, brown, rubs clean; of KCN, negative; of FeCl3, negative. The white mineral is hard and is negative to HNO3, HCl, and FeCl3.
If further
investigation of these nickel-cobalt arsenides should establish them as new
species, it would be desirable to retain the names mohawkite and keweenawite
which Koenig gave to certain intimate mixtures of arsenides from these fissures.
HALOIDS
OXIDES
Quartz (SiO2) occurs as an original. mineral in the felsites and quartz porphyries. It is present in practically all lodes and fissures throughout the district as a vein filling, as a. filling of vesicles, and replacing other minerals and rock. It is perhaps most abundant in the Evergreen lodes and the Isle Royale lode, but it is plentiful in others, as the. Baltic and Pewabic and locally in the Osceola and Kearsarge. In the fissures it is particularly abundant with the arsenides. Quartz was formed through the greater part of the ore period but most abundantly in the middle portion. It replaced the rock minerals and some of the earlier ore minerals and was in turn replaced by some of the later minerals. Chalcedony occurs as lining of vugs in the Pewabic lode and in amygdules in the Kearsarge lode. It has replaced zoisite and calcite in many places.
Cuprite (Cu2O) occurs in well-formed crystals at the Indiana mine as an oxidation product of copper; it occurs similarly at the Algomah mine, where it is largely altered to tenorite. The copper in many of the mine openings is colored with a film of cuprite. In places this film has clearly formed after the mines were opened. In other places, especially in the higher levels, it probably formed before the mines were opened.
Tenorite (melaconite, CuO), the black oxide of copper, is especially abundant at the Algomah mine and at Copper Harbor and doubtless occurs elsewhere. It is a product of oxidation of other minerals.
Atacamite (Cu2Cl(OH3)) occurs in well-formed radiating crystals as an oxidation product at the Algomah mine.
Hematite (Fe2O3) is apparently a primary mineral in the felsite and in the felsite pebbles of the conglomerates. The abundant hematite in the red tops of the lava flows is believed to have been formed from magnetite and the iron silicates or from the glassy portion of the tops of flows, or possibly in part crystallized directly during the solidification and cooling of the flows. Deeper in the flows the hematite has formed from magnetite and olivine, likewise probably during solidification. This process is fully discussed in the section on oxidation of lava tops (p. 38). Hematite occurs in small amount in some of the veins that contain sulphide and arsenide. In these it is usually associated with ankerite. In the conglomerate specular hematite is present in some of the iron-rich porphyry boulders. Specular hematite also occurs sparingly in fractures in both the conglomerate and annygdaloid lodes. It is included in the red feldspar of the lodes. Characteristically hematite has been destroyed near copper, and the iron has been either recombined or removed. The formation of hematite mainly preceded the ore-forming period. During that period a little was formed, probably by the recrystallization of earlier hematite.
Ilmenite (FeTiO
3) occurs as a primary mineral in traps. It has been altered in places to hematite and titanite.Brucite (MgO
2H2) occurs in vugs in the isle Royale and Kearsarge lodes. It is a late mineral of the ore period. In the one specimen from the Kearsarge lode it has replaced laumontite. In the Isle Royale mine it was replaced by sericite Magnetite (Fe3O4) was originally an abundant primary mineral in nearly all the flows, but it has been entirely altered to hematite in the tops of the flows and throughout some flows. This alteration is discussed in connection with oxidation of lava tops (p. 34). Magnetite is present as grains in some of the conglomerates, as the Great conglomerate and the Houghton conglomerate, but in most of them it has been altered to hematite or limonite. It is rarely present in the fissure veins. The magnetite of the flows is titaniferous, and hematite, titanite, and rutile were formed from it on alteration.Rutile (TiO
2) occurs in basalt as an alteration product of titaniferous iron ore, and it occurs also in the felsites, where it was possibly derived from biotite.Manganite (Mn
2O3·H2O) occurs as small crystals in vugs in the Calumet & Hecla conglomerate. At the Manganese mine, near Copper Harbor, it occurs with other manganese minerals as a "vein," essentially coinciding with an amygdaloid a short distance below the Great conglomerate. The "vein" was opened for a few hundred feet along the strike, and some ore was shipped from the mine.Pyrolusite (MnO
2) occurs at the Manganese mine, near Copper Harbor.Limonite (2Fe
2O3·3H2O) occurs sparingly on weathered surfaces of trap. It is present in the conglomerates, probably as alteration product of magnetite. It is rarely seen in the amygdaloid lodes.CARBONATES
Calcite (CaCO
3) is perhaps the most widespread mineral of the ore-forming period. It occurs as crystals in open cavities and replaces other minerals and is everywhere present in veins, amygdaloids, and conglomerates. It was formed from about the middle to the end of the ore-forming period, and therefore it has replaced many minerals and has itself been replaced by several. It occurs in the pegmatitic lenses in some of the thick traps. It is also being formed in the present mine workings where water is dripping in the upper levels and around masses of copper in the walls of openings in the deep levels. In many places crystals of calcite inclose small crystals of copper.Ankerite ((Ca,Mg,Fe,Mn)CO
3, composition variable) is common in the sulphide and arsenide veins and is also abundant in parts of the Baltic lode as a lode mineral. In sulphide veins it commonly occurs next to the walls, with sulphides in the center. The same relation is shown in the Baltic lode, where the carbonate lines cavities and incloses sulphide. It has also replaced the lode rock. Where it is associated with calcite the calcite is later. The mineral apparently was formed at intermediate to late stages in the ore period and is closely associated with sulphides and arsenides.Malachite (Cu
2(OH)2·CO3) was noted particularly at the Algomah mine as an oxidation product. It is doubtless present in small amount at other places.SILICATES
The plagioclase series of feldspars, consisting of isomorphous mixtures of albite (NaAlSi
3O8) and anorthite (CaAl2Si2O8), is represented by plagioclase of intermediate composition, which forms an abundant original constituent of all the flows. Albite is present in the felsites. The feldspars of the flows underwent little alteration during the oxidation of the flow tops, the microlites of plagioclase being still remarkably fresh. Plagioclase was, however, one of the earliest minerals to be attacked by the ore solutions, though locally it remains remarkably fresh in otherwise altered rock. The feldspar was commonly replaced by chlorite, epidote, zoisite, sericite, or calcite, but prehnite or any of the later minerals have replaced plagioclase. Albite in relatively small amount is present in many places as one of the later minerals of the ore-forming period.Orthoclase (KAlSi
3O8) occurs in three distinct ways-as an original mineral of the felsite, as an early mineral of the ore period (red feldspar), and as adularia, a late mineral of the ore period. The original orthoclase of the felsites occurs both as phenocrysts and in the groundmass. Red feldspar, probably of variable composition, is abundant in several of the lodes and is also present in many fissures. It is especially abundant in the Butler lode and adjacent lodes, in the Superior lode, and in parts of the Kearsarge and Osceola amygdaloids. It is present but not abundant in the conglomerates. It occurs in fissures and amygdules, and it replaces the rock, especially in the Evergreen and succeeding lodes but to some extent in most of the other lodes. It was one of the earliest minerals of the ore period and was replaced by chlorite, epidote, and later minerals.Copper occurs with red feldspar, but the association is not notably close. The feldspar, like many minerals of the ore period, is far more widespread than copper.
Adularia occurs to a minor extent in lodes and fissures as well-formed white to pinkish crystals, usually in open spaces. It was apparently a relatively late mineral of the ore period.
Pyroxene (Ca(Mg,Fe)Si
2O6) is an abundant primary mineral of all the basic flows. Its usual alteration is to hematite and serpentine. In places it. is stained with limonite. It was rather resistant to alteration by the ore solutions, but where the alteration was intense it was replaced, like the other rock minerals.Hornblende was noted in the Greenstone flow associated with biotite.
Olivine (Mg,Fe)
2SiO4) was an early mineral to crystallize in most of the lava. flows. It is now mostly altered, characteristically to serpentine or bowlingite and hematite. These minerals were in places further altered to others. Fresh olivine was observed only in the coarse phases of the Greenstone flow.Zircon (ZrSiO
4) occurs as a primary mineral of the felsites.Pumpellyite (6CaO·3Al
2O3·7SiO2·4H2O), provisionally called zoisite, occurs in bluish-green lath-shaped crystals or needles, in most places in radiating groups. It is this mineral that gives the characteristic bluish-green color to much of the lode material in the mines in the south end of the district. It is near zoisite in composition but differs from zoisite crystallographically. Doctor Palache69 has described this mineral in detail.
Analysis of
pumpellyite
[Helen E.
Vassar, analyst]
| Analysis | Molecular ratio | ||
| SiO2 | 37.18 | 0.616 | 0.618=7 |
| Al2O3 | 23.50 | 0.260 | 0.293=3 |
| Fe2O3 | 5.29 | 0.033 | |
| FeO | 2.09 | 0.029 | 0.524=6 |
| MgO | 3.18 | 0.079 | |
| CaO | 23.08 | 0.411 | |
| MnO | 0.13 | 0.002 | |
| Na2O | 0.19 | 0.003 | |
| K2O | Trace | ||
| H2O+ | 6.28 | 0.349 | .349=4 |
| H2O- | 0.06 | ||
| 100.97 | |||
The mineral is abundant in all the amygdaloid lodes and in all the fissures. It is present but not usually abundant in the conglomerates.
It is especially conspicuous in the Isle Royale lode and in the Evergreen and succeeding lodes, but hardly more so than in parts of the Baltic and Pewabic lodes. It occurs in open cavities and replaces the rock. In many places pumpellyite with quartz has partly replaced large volumes of rock. It commonly followed epidote in formation but preceded copper. It replaced the earlier minerals of the ore period, such as chlorite and epidote, and in turn was replaced by the later minerals, including copper. It is not usually abundant in highly oxidized rock, such as the top of the Kearsarge amygdaloid. It is closely associated with copper in many places, but apparently, like many of the gangue minerals, is far more widespread in occurrence than copper. The lodes of the Pewabic series in particular in numerous places are rather highly pumpellyitized where they are not known to contain abundant copper.
Epidote (composition variable, approximately HCa2 (Al,Fe)3Si3O13), a yellow-green mineral, is every where abundant in both veins and lodes, being perhaps especially abundant in the Evergreen and succeeding lodes of that series and in the Isle Royale lode. It is also abundant locally in the Calumet & Hecla conglomerate. Epidote characteristically has replaced the rock minerals, though it has also been formed in openings. It commonly first replaced plagioclase but eventually replaced much of the rock in mass. In the Calumet & Hecla conglomerate it replaced sandstone lenses. In the Kearsarge lode it is relatively. abundant in the upper, highly oxidized portion as contrasted with the lower, less oxidized portion. The iron entering the epidote was apparently derived from the hematite of the rocks. Epidote is one of the early minerals of the ore-forming period, in the main preceding copper, but it also appears to have been formed to a slight extent later than copper, as it cuts anhydrite and sericite - in the Isle Royale mine, for instance. Epidote is in many places closely associated with copper, but, like chlorite and calcite, it is far more widespread than the commercially valuable copper minerals. Tourmaline occurs in felsite fragments in the conglomerates.
It is unrelated to the copper mineralization.
Natrolite (Na
2Al2Si3O10·2H2O) occurs with analcite and adularia at Copper Falls.Analcite (NaAlSi
2O6·H2O) occurs commonly as a vein mineral, more rarely in vugs in the lodes. It occurs in vugs as white, well-formed crystals, in many of which the centers are partly dissolved. In veins it rarely shows crystal form and is pinkish in color. It is one of the late minerals of the ore-forming period but in places was followed by saponite and calcite. It is a common mineral but nowhere abundant. Most occurrences were noted in the Kearsarge lode. Stilbite (CaAl2Si6O16·6H2O) occurs in the Osceola amygdaloid and at Copper Falls.Heulandite (CaAl
2Si6O16·5H2O) is one of the rarest zeolites of the district. Thomsonite (CaAl2Si2O8·2½H2O) occurs mainly as a filling of amygdules but also in veins. It is usually not closely associated with copper.Apophyllite was observed in the St. Clair, Robbins, Cliff, and North American fissures. It is one of the latest minerals.
Chlorastrolite occurs as amygdule filling in flows on Isle Royal. It is worn from the matrix by wave action, and the stones are gathered on the beaches.
Faujasite (H
2(CaNa2)Al2(SiO3)5·9H2O) from Copper Falls is in the Whitney collection.Datolite (H
2Ca2B2Si2O10) occurs in both fissures and lodes. It is probably present in most of the amygdalaid lodes, but it is relatively abundant in the Evergreen and succeeding lodes, especially at the Mass Mine, in parts o£ the Kearsarge and Osceola lodes, in parts of the Pewabic lodes, and in the Ashbed lodes in Keweenaw County. It has not been noted in the conglomerate lodes. It occurs in fissures at many places and is abundant at the Copper Falls (Owl Creek) and Petherick fissures, where locally at least it formed the main gangue mineral. Datolite forms both well developed crystals and dense porcelanic masses. The crystalline datolite occurs in both fissures and lodes. It may be pink from included copper, and where the crystals are small they occur as a loose, crumbly granular aggregate. The porcelanic variety occurs characteristically as irregular spheroidal masses with a botryoidal surface. (See pl. 66.) These masses of datolite commonly contain many small specks of copper, which are usually concentrated near the surface of the mass, though less commonly the concentration is toward the center. This variety has been found most abundantly in the higher levels of the Pewabic lodes but is present in other lodes. Datolite apparently was one of the intermediate to later minerals of the ore period.Prehnite (Al
2(SiO4)3Ca2H2) commonly occurs in radiating crystal aggregates but in the veins especially may appear massive. Much of it has a light apple-green tint, but it varies greatly in color, some of it being nearly black. It is abundant in veins throughout the district. It is present in most of the amygdaloids, where it has filled vesicles or replaced earlier minerals or rock. Locally it is abundant in the amygdaloids, as in the Osceola lode, where it largely replaced basic sediment. It is rare in the conglomerates. Prehnite was formed mainly as an intermediate mineral of the ore period, but some was later. In the veins it is very commonly associated with copper, which in many places is disseminated through it in minute grains. The highly prehnitized amygdaloid, however, is not usually high in copper. Prehnite has been replaced by the later minerals.Sericite (muscovite, Al
3KH2Si3O12) has mainly replaced rock and earlier ore minerals but also filled open spaces. It is most abundant as a replacement mineral in the walls of the arsenide and sulphide fissures and in some of the lodes, as the Isle Royale and locally in the Baltic. In the Isle Royale lode it is rather abundant as a soft talcose mineral replacing fragmental amygdaloid. Sericite seems to have been formed from a moderately early stage in the ore forming period to a rather late stage.Analysis
of sericite from Isle Royale amygdaloid
[Helen E. Vassar,
analyst]
| SiO2 | 50.27 | K2O | 9.61 |
| Al2O3 | 27.71 | Na2O | 0.15 |
| FeO | 0.534 | H2O+ | 4.68 |
| Fe2O3 | 1.87 | H2O- | 1.44 |
| MnO | 0.03 | CO2 | 0.76 |
| CaO | 1.06 | TiO2 | Trace |
| MgO | 2.39 |
Biotite (Al
2Mg2KHSi3O12, with variable proportions of ferric and ferrous iron) is a rare primary constituent of some of the basic lava flows, such as the Ashbed group and the Greenstone flow.Chlorite (delessite, H
10(Mg,Fe)4Al4Si4O22, variable) occurs abundantly as a vesicle filling and as a vein mineral, and it has also replaced rock minerals. It is particularly abundant in some of the veins and has replaced the adjacent wall rocks. In the amygdaloids it is especially conspicuous in the less oxidized parts, such as the lower portion. In the Calumet & Hecla conglomerate it has replaced some of the iron-rich pebbles, where it is associated with copper. At White Pine it surrounds hydrocarbon and has been replaced by copper. Some chlorite was probably formed during the cooling and solidification of the laves. It was an abundant early mineral of the ore-forming period but apparently continued to form till late in that period. It replaced many minerals and was replaced by several, being very commonly replaced by copper.Serpentine (Mg
3H4Si2O9) is a common alteration product of olivine in the traps, and it also occurs in cavities in the amygdaloid lodes. The serpentine of the former type was earlier than the ore period; that of the latter type was formed late in the ore period.Bowlingite is the common alteration product of olivine in many of the flows. It is resistant to alteration but has been replaced in places by prehnite and calcite.
Saponite (Al
2O3·MgO·10SiO2·15 or 16H2O) occurs in several of the lodes, where it has filled vesicles, replaced other minerals, and formed as a crust on other minerals. It was noted most abundantly in the Kearsarge lode. It is apparently a late mineral of the ore period.
Analyses of saponite
from the Kearsarge lode
[Helen
E. Vassar, analyst]
| South Kearsarge mine |
Ahmeek mine |
|
| SiO2 | 42.78 | 42.99 |
| Al2O3 | 6.44 | 6.26 |
| Fe2O3 | 1.27 | 2.57 |
| FeO | ||
| MgO | 24.78 | 22.96 |
| CaO | 2.35 | 2.03 |
| MnO | 0.12 | 0.11 |
| Na2O | 0.75 | 1.04 |
| K2O | Trace | Trace |
| H2O+ | 7.90 | 6.85 |
| H2O- | 13.96 | 13.65 |
| 100.35 | 100.29 |
Kaolin (H4Al2Si2O9) occurs in the Isle Royale mine on laumontite and in the Kearsarge lode as a crust on red feldspar. It is probably a late mineral of the ore-forming period. A little occurs clouding plagioclase that is believed to have been formed when the lavas were exposed at the-time of extrusion.
Chrysocolla (CuSiO
3·2H2O) occurs as an oxidation product at the Algomah mine and the Allouez conglomerate mine and at Copper Harbor.Titanite (CaTiSiO
5) is rather abundant in all the flows as an alteration product of olivine, pyroxene, or titaniferous magnetite. In highly altered lode rock it is common as a residual mineral.PHOSPHATES
Apatite (Ca5(PO4)3F or Ca5(PO4)3C1) occurs rather sparingly as needles or prisms in the felsite pebbles of the conglomerates and in the lava flows. It is an early original mineral of the rocks.SULPHATES
Barite (BaSO
4) occurs most commonly in veins, in many places with sulphide. It also occurs in several of the amygdaloids and in the iron-rich pebbles of the Calumet & Hecla conglomerate. At the White Pine mine it is a vein mineral and a cement in the sandstone. It was among the late minerals, though followed in places by saponite, adularia, and datolite. It is widespread but nowhere abundant.Anhydrite (CaSO
4) occurs in vugs and has replaced rock in the Isle Royale mine. It was also collected from the dump of the Mass mine. It seems to be of the late ore-forming stage but is veined in the Isle Royale mine by laumontite and epidote.Gypsum (CaSO
4·2H2O) occurs sparingly at numerous places, both in fissures and in the lodes. It is nowhere abundant. It appears to be a late mineral. In the Isle Royale mine it seems to have been formed from anhydrite. In the Victoria mine it has formed in puddles of salt water that stand in a crosscut.MOLYBDATES AND TUNGSTATES
Powellite (Ca(Mo,W)O
4) has been reported from the Isle Royale mine, and a few well-formed crystals have been found in the Calumet & Hecla conglomerate. The mineral is said to contain both molybdenum and tungsten.HYDROCARBON
A dark hydrocarbon is rather plentiful in the Nonesuch lode at the White Pine mine, where it occurs in fissures and as a cement in the sandstone. In places it is surrounded by and was evidently earlier than copper. The hydrocarbon in the fissures contains abundant copper.
NOTES
67 Koenig, G. A., On artificial production of crystallized domeykite, algodonite argentodomeykite, and stibiodomeykite: Am. Philos. Soc. Proc., vol. 42, pp. 219-237, 1903.
68 Koenig, G. A., op. cit.
69 Palache, Charles, and Vassar, A. E., Some minerals of the Keweenaw copper deposits; pumpellyite, a new mineral; sericite; saponite: Am. Mineralogist, vol. 10, pp. 472-473, 1925.
Collector's Corner Previous section Table of Contents Next section