| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
Volume 13, pages 334-340, 1928 LARSENITE, CALCIUM-LARSENITE AND THE ASSOCIATED MINERALS AT FRANKLIN, NEW JERSEY C. PALACHE, L. H. BAUER, AND H. BERMAN A preliminary description of larsenite and calcium-larsenite, new lead silicates, has recently been published.1 There is little to add to this description of the new minerals except details of the crystal form of larsenite. But the paragenesis is complex and unusual and seems worthy of somewhat full description. The first specimens were found on the picking table but the place of their occurrence in the mine was afterwards located in the North end, 20 feet above the 400' level in the 1080 top-slice. A second locality of calcium-larsenite has been found, also in the North end but on the 1100' level in 80 pillar. The specimens in hand are in part veins cutting ore, in part replacements of massive, rather coarse willemite-franklinite ore. The calcium-larsenite is all of the latter type and appears to have replaced willemite or in places hardystonite with which it is intimately associated. In the locality on the 1100' level, calcium-larsenite is similarly related to a mixture of glaucochroite and hardystonite with franklinite. The veins are sharply bounded against the granular ore and vary in their contents from place to place. The earliest mineral to form is a massive pink garnet, identical in appearance and refractive index (1.885) with a garnet from another specimen, shown by partial analysis to be andradite. Following this is a coating of hodgkinsonite of the same pink color as the garnet where it is massive. Most surfaces of the open veins show crystals of hodgkinsonite of a pale pink to almost colorless variety, the crystals rather indistinct and with rounded faces. In several specimens devoid of lead silicates the only minerals later than hodgkinsonite are calcite in slender needles or clear prismatic crystals; botryoidal coatings of smithsonite; granular orange colored zincite; and a last coating of snow white needles of willemite forming delicate rosettes. In other parts of the open veins hodgkinsonite was followed by an abundant layer of crystalline clinohedrite . with which the slender needles and plates of larsenite are contemporaneous. A description of the form and properties of this clinohedrite will be found on page 303. A few spots of dull white massive roeblingite were found in one of the specimens covering clinohedrite crystals. Larsenite is far less abundant than calcium-larsenite and in most of the specimens the two are not associated but in two cases crystals of larsenite occur in cavities in the walls of which calcium-larsenite is present. In one specimen of calcium-larsenite an area of that mineral an inch across is surrounded by a zone of grayish bementite and about this in turn is a border of a dark brown glassy substance determined optically as neotocite. The latter mineral has been described as the alteration product of bementite2 and this would appear to be the case here as well. TABLE 1. COMBINATIONS OF FORMS ON LARSENITE Numbers in squares indicate number of faces present
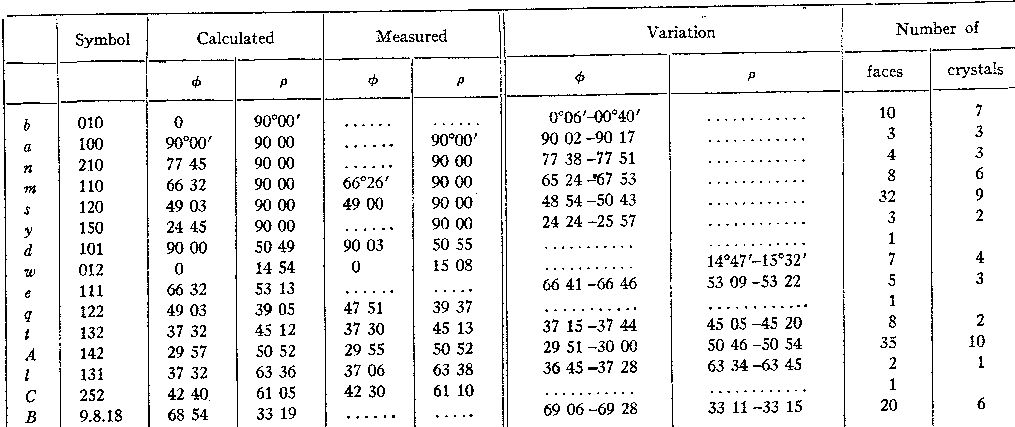
In Table II are the calculated position-angles and the measured angles of each form. TABLE II. ANGLE TABLE OF LARSENITE |
|
LARSENITE. CRYSTALLOGRAPHY. The crystals of larsenite are slender needles ten to twenty times as long as they are in cross section, forming an interlacing network in vein cavities. Many of them reach from wall to wall and show no termination but a few stand with one end free and could be detached for measurement. In two specimens only it has the form of thin lustrous plates. Ten crystals were studied in detail and the distribution of forms upon them is shown in TABLE I. The axes were calculated from forty faces of the five forms (120), (142), (132), (111) and (131) with the following result: a : b : c = 0.4339 : 1 : 0.5324 The prism zone is generally rounded by striation, one crystal only showing a pronounced flattening parallel to the pinacoid (010). The prism (120) to which .the cleavage is parallel is always present and gives good reflections while (110), (210) and (150) are line faces only. The termination is generally dominated by the pyramid (142) the faces of which are excellent and give consistent readings. The calculation of the axes is chiefly based on this form and the prism (120). This pyramid was at first selected as the unit form but it was found that the relation to the chrysolite group of minerals was much better satisfied by the position here adopted. The obtuse edge of this pyramid is generally truncated by a pair of narrow faces of the pyramid (9.8.18) which is close to the position of the much simpler form (112) but deviates consistently from it.
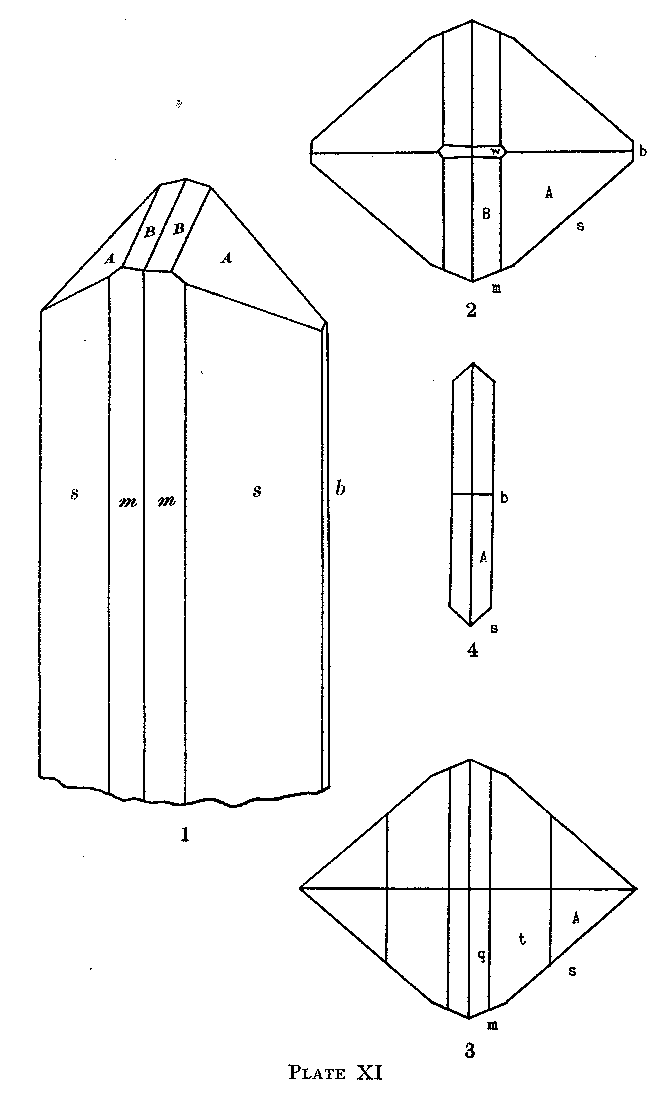
Plate XI shows crystals of larsenite. Figure 1 shows the prevailing habit. In figure 2 the brachydom is added. Figure 3 shows a habit observed on but a single crystal. The platy habit is illustrated in figure 4. Forms not shown in the figures are very subordinate in development, generally occurring as line faces only. The needles are very slender so that the terminal faces are in all cases exceedingly minute. Because, however, of the high lustre of the mineral they give good measurements despite their minute area. PLATE XI |
|
EXPLANATION OF PLATE XI Fig. 1. Larsenite. Drawing of a crystal showing the normal termination, with the forms b(010), m(110), s(120), A(142) and B(9.8.18). Fig. 2. Larsenite. Drawing in plan of a crystal showing the forms of figure 1 and in addition w(012). Fig. 3. Larsenite. Plan of a crystal showing the forms m(110), s(120), q(122), t(132), and A (142). Fig. 4. Larsenite. Plan of a crystal of tabular habit showing the forms b(010), s(120) and A(142). PHYSICAL PROPERTIES. The specific gravity of larsenite, determined with the pycnometer on a very small (.075 gr.) pure sample is 5.88. The analyzed sample gave, by the same method, a value of 5.67. This sample was found to contain 9.26 per cent. of clinohedrite with a specific gravity of 3.33 and by calculation this gave a value for larsenite of 5.91. The average of the two determinations, 5.90, was taken as the true value but it is evident that the determination requires confirmation on a larger pure sample. Larsenite is about 3 in hardness, has the adamantine lustre characteristic of lead compounds and the platy variety has a somewhat pearly lustre on the surface of the plates. The indices of refraction had to be determined in melted media and may be slightly incorrect because of the heating involved which caused a slight yellowing of the white mineral. With the above exceptions, nothing has been added to the physical data presented in the preliminary paper3 for either larsenite or calcium-larsenite. The same holds true of the chemical data there presented. The analyst, Mr. Bauer, gives the following information as to the method of analysis and chemical properties of the minerals. "Both minerals dissolve readily in dilute nitric acid giving gelatinous silica. In the oxidizing flame of the blow-pipe larsenite fuses easily to a white enamel, while the unfused portion turns yellow, when further heated in the reducing flame the mass turns black indicating the reduction of the oxide to metallic lead. The calcium-larsenite while not fusing quite so readily shows the same tests in the blowpipe flames. About .6 grams of a purified sample of larsenite was obtained for complete analysis. After taking .1056 grams for a Penfield water determination the balance was weighed into two portions for complete analysis. First the silica was obtained from a double. evaporation in nitric acid solution, the filtrate from the silica was evaporated to dryness to expel all traces of nitric acid, and the residue dissolved in an accurately adjusted 1% HCl solution. This acidity is essential in order to best precipitate all traces of lead with H2S. The PbS was filtered off, redissolved and in turn converted to lead sulfate and lead chromate. The filtrates from the lead sulfide and lead sulfate were combined and taken to dryness. The RO group was then removed by precipitation with bromine and ammonia and weighed as R2O3. The weight obtained from (a) was redissolved and the FeO determined by the standard sulphocyanate method. In (b) the MnO was determined by oxidation with ammonium persulfate and subsequent titration with a standard sodium arsenite solution. The lime and magnesia were found respectively by the well known oxalate and pyrophosphate methods. In the filtrate from the magnesia determination the zinc was found by the acid ferrocyanide method. The, same procedure was followed with the calcium-larsenite analysis. Due to its fluorescence under the iron-arc a pure sample for analysis was readily obtained, by hand picking." The analytical results are as follows: LARSENITE
1 Substituted result from (b). CALCIUM-LARSENITE
3 Discarded in mean.
The group relationship of these minerals is as follows:
It is interesting to note in view of the discovery of these lead silicates that in both analyses of glaucochroite (ante p. 308) some lead is reported, showing possibly the presence of the larsenite molecule. NOTES 1 Am. Mineral., 13, 142, 1928. 2 Pardee, J. T., Larsen, E. S., and Steiger, G. Bementite and Neotocite from Western Washington. Jour. Wash. Acad. Sci., 11, 25, 1921. 3 See ante p. 334.
|