ABSTRACT
The first measurable triplite crystals to be recorded were found in the Mica
Lode pegmatite, Eight Mile Park, Fremont County, Colorado. Two other new
triplite localities are the School Section pegmatite in the same district and
another pegmatite in El Paso County, Colorado. The triplite occurs in
hydrothermal replacement units along the footwall sides of core pods. On the
basis of crystallographic measurements of four crystals and x-ray examination of
an oriented section, triplite is monoclinic, prismatic -2/m(?), with ao=11.90,
bo=6.48, co =9.92, β=105°53'. The space group is I 2/m? The optical properties
and specific gravity of the Mica Lode material correspond closely with those of
triplite from the 7U7 Ranch, Arizona, and it is believed that the chemical
composition is also similar.
INTRODUCTION
While engaged in a study of the pegmatite deposits of Eight Mile Park, Fremont
County, Colorado, the junior author obtained a number of large, rudely faced
crystals of triplite. The crystallography and x-ray structure of these crystals
were studied subsequently by the senior author in the Mineralogical Laboratory
of Harvard University. In view of the fact that the material represents the
first recorded find of triplite, crystallized sufficiently well for measurement,
it is believed that a detailed description is warranted. The senior author has
made all the crystallographic and x-ray measurements and calculations. Geologic
and paragenetic information has been supplied by the junior author.
Responsibility for other parts of the paper is assumed jointly. Thanks are due
to the late Dr. Charles Tozier of the Committee on Visual Education of Harvard
University for the photograph of the triplite crystals.
GENERAL GEOLOGY
The geology of the Eight Mile Park pegmatite area has been studied by the junior
author (Heinrich, 1947). The area is a 25-square mile plateau roughly bisected
by the Royal Gorge of the Arkansas River. It is underlain by pre-Cambrian rocks
of three main types: (1) Pikes Peak granite, (2) Idaho Springs schist, and (3)
injection gneiss formed by lit-par-lit intrusion of schist by granitic material.
The northwestern unit is granite which is bounded on the southeast by a narrow
northeast-trending belt of Idaho Springs schist. The southeastern part of the area is underlain
by injection gneiss.
Moderately to steeply dipping pegmatite sills and concordant lenses are locally
abundant in the Idaho Springs formation near the contacts with the Pikes Peak
batholith. They attain a maximum length of 3000 feet and a thickness of 800
feet. Within the granite, also near the margins of the batholith, occur large,
flat-lying, discoidal bodies of pegmatite that transect the primary igneous foliation. Two of the three larger pegmatite
deposits, the Meyers Quarry body and the Mica Lode, are in schist, and the
third, the School Section deposit, occurs in granite. Triplite was found in the
Mica Lode and School Section pegmatites. The location of these deposits is shown
in Fig. 1. The Mica Lode is in the NE 4, SW. 4, sec. 14. T. 185., R. 71 W. and
the School Section quarry is in the southeast corner of Sec. 16, T. 18 S., R. 71
W.
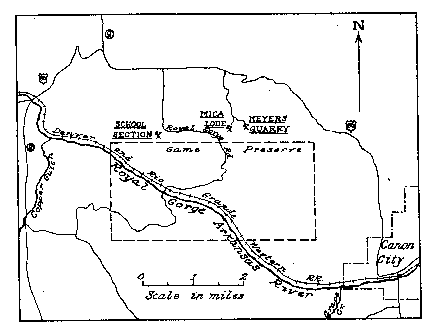
FIG. 1. Index map showing location of Mica Lode and School Section pegmatite
deposits, Eight Mile Park, Fremont County, Colorado.
OCCURRENCE
Triplite in rounded, anhedral masses as much as six inches in length is abundant
locally in the School Section pegmatite, but no crystals were obtained from this deposit. Associated minerals are muscovite, plagioclase,
black tourmaline, and beryl. The plagioclase is of two types: pink
oligoclase-albite and white cleavelandite. These minerals are concentrated in
units of restricted size which underlie flat-lying to horizontal core pods. The
core pods, or central zones within the pegmatite, are composed of massive white
quartz and crystals of pink microcline as much as six feet across. The zonal
structure appears to have formed by direct crystallization from the pegmatite
magma. The triplite-bearing units, which are clearly secondary, probably formed
by hydrothermal replacement of parts of the cores and parts of the adjoining
graphic granite quartz-muscovite zone.
The geologic relations at the Mica Lode are similar, except that the
microcline-rich core is a single large unit that dips steeply northwest in
conformity with the general attitude of the pegmatite. The footwall half of the
core has been extensively replaced by abundant pink oligoclase-albite and
muscovite. Beryl, with which the triplite is associated, is abundant locally in
this replacement unit. Triplite pods as much as two feet long were observed in
place, and about 10 tons of the material has been hand-cobbed and stockpiled.
The crystals studied were obtained from the stockpile.
Some nodules of triplite are corroded and veined by albite and muscovite. At the
School Section deposit veins of black tourmaline transect the phosphate. In this
respect the material is similar to that found at Chatham, Connecticut. This
occurrence has been described by Shannon (1921) who states (p. 445), "Intergrown
intimately with the triplite are muscovite and fine-grained deep-blue
tourmaline, the last surrounding the triplite in a crust and penetrating it
along cracks . . . ."
In 1945 Dr. Chalmer J. Roy of Louisiana State University sent to the
Mineralogical Laboratory of Harvard University a specimen of triplite from El
Paso County, Colorado. According to Dr. Roy the mineral occurs in a pegmatite in
the southeast corner of sec. 9, T. 16 S., R. 67 W., where it is associated with
quartz, microcline, and muscovite.
OCCURRENCE OF TRIPLITE IN THE UNITED STATES
Data on the occurrences of triplite in the United States are summarized in Table
1.
TABLE 1. TRIPLITE OCCURRENCES IN THE UNITED STATES
| No. |
Locality |
Reference |
Occurrence |
Probable Origin |
| 1 |
Stoneham, Maine |
Kunz, 1884 |
pegmatite |
hydrothermal replacement |
| 2 |
Auburn, Maine |
Bastin, 1911 |
pegmatite |
hydrothermal replacement |
| 3 |
Chatham, Conn. |
Shannon,1921 |
pegmatite |
hydrothermal replacement |
| 4 |
Branchville, Conn. |
Dana, 1892 |
pegmatite |
hydrothermal replacement? |
| 5 |
School Section, Fremont County, Colo. |
Wolfe and Heinrich, 1947 |
pegmatite |
hydrothermal replacement |
| 6 |
Mica Lode, Fremont County, Colo. |
Wolfe and Heinrich, 1947 |
pegmatite |
hydrothermal replacement |
| 7 |
El Paso County, Colo. |
Wolfe and Heinrich, 1947 |
pegmatite |
? |
| 8 |
7U7 Ranch, Arizona |
Hurlbut, 1936 |
pegmatite |
?; described as a segregation |
| 9 |
Mt. Loma and two nearby localities, Arizona |
Hurlbut, 1936 |
pegmatite |
?; described as segregations
|
| 10 |
White Pine Co., Nevada |
Hess and Hunt, 1913 |
high temperature vein |
hydrothermal deposition |
CRYSTALLOGRAPHY
Four crystals from the Mica Lode pegmatite were examined and measured by means
of the contact goniometer. The two large ones, shown in Figs. 2 and 3, proved
very satisfactory for contact measurement purposes and yielded moderately
reproducible results. The dimensions of these two crystals, measured in the directions of the a, b, and c axes, are 9
X 9 X 12 cm. and 9 X 12 X 9 cm. The other two crystals, which are less well
developed, have maximum dimensions of 9 cm. The quality of the angular readings
on the smaller crystals is inferior to that for the larger ones.
Quality of measurements and important measured and calculated values for the
four crystals are given in Table 2. No elements have been derived from these
measurements, because it is believed that the elements derived from x-ray values
are more satisfactory. The correlation with the structural cell was accurately
determined by cutting an oriented fragment from crystal 1, from which rotation
and weissenberg data were obtained.
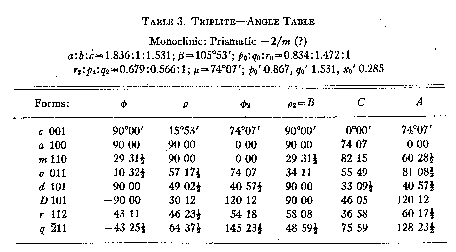
With the elements derived from an x-ray examination of an oriented crystal
fragment, the following angle table (Table 3) was prepared. All of the forms
listed may be considered certain. Although {112} was observed but once, it was
clearly found and in good position.
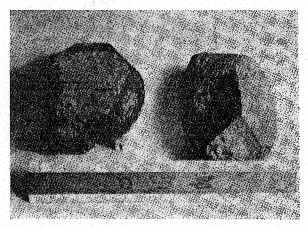
FIG. 2. Photograph of triplite crystals (crystals 1 and 2), from the Mica Lode
pegmatite, Fight Mile Park, Fremont County, Colorado.
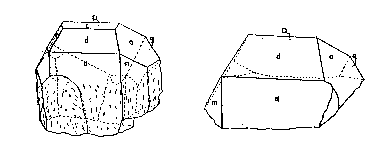
FIG. 3. Crystals of triplite from the Mica Lode pegmatite; left--crystal 1,
right-crystal 2.
It is interesting to note that all of the observed forms have indices that
conform to the body-centering criteria of h+k+l=2n, and the relative importance
of the forms is in simple relationship to the spacing of the planes in the
centered lattice.
Full development of a prismatic form was not observed. The crystal class is not
certain; but, since a plane of symmetry was apparent on crystals 1 and 2, the
class must be domatic-m or prismatic -2/m. The latter is assumed here for lack
of evidence to the contrary.
TABLE 2. SUMMARY OF CRYSTALLOGRAPHIC DATA
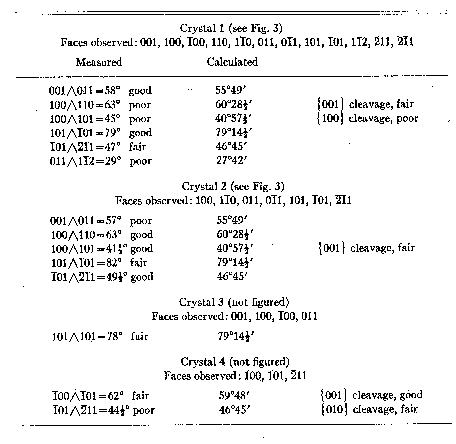
TABLE 3. TRIPLETT-ANGLE TABLE
X-RAY DATA
In order to correlate the geometrical crystallography with the x-ray data of
Richmond (1940) an oriented section was cut from crystal 1, and rotation,
0-layer line, and 1-layer line pictures were taken about the [010] axis, with
copper radiation. The solution of these pictures gave the following results:
| |
Wolfe-Heinrich |
Richmond |
|
Space Group |
I 2/m |
P 21/a |
| ao |
11.90 |
12.03 |
| bo |
6.48 |
6.46 |
| co |
9.92 |
10.03 |
| β |
105°53' |
105º42' |
|
Measured gravity |
3.64 |
3.84 |
|
Calculated gravity |
3.64 |
3.94 |
| Mg:Mn:Fe= |
2:3:1 |
Fe:Mn=1:8 |
Richmond's original pictures were reexamined, and the space group is definitely
body-centered.
OPTICAL PROPERTIES
The optical properties of the Mica Lode triplite are as follows:
| Orientation |
n |
Pleochroism |
| X^a=57° |
1.643±0.003 |
reddish brown |
| Y =b |
1.647 |
yellow |
| Z^c=-41° |
1.668 |
reddish brown |
(+); 2V=25° (measured); r>v, moderate
Table 4 compares the optical properties of the Colorado triplites.
TABLE 4. OPTICAL PROPERTIES OF COLORADO TRIPLITES
| |
School Section |
Mica Lode |
El Paso County |
| nX |
1.671±.003 |
1.643±.003 |
1.648±.003 |
| nY |
1.681 |
1.647 |
1.652 |
| nZ |
1.686 |
1.668 |
1.672 |
| 2V |
moderate |
40° |
55° |
| Sign |
(-) |
(+) |
(+) |
CHEMICAL COMPOSITION
The optical properties of the Mica Lode triplite are comparable to those
determined by Hurlbut (1936) for the mineral from the 7U7 Ranch, which contains nearly 12% MgO and has an Mg: Mn: Fe ratio of 2:3:1. The
specific gravity for triplite of that composition as calculated with our x-ray
data is 3.64. Several gravity determinations, made by means of the Berman
balance on pure fragments of the Mica Lode triplite, gave an average value of
3.64. A qualitative test indicated the presence of relatively large amounts of
magnesium in this triplite.
Complete analyses of both the Mica Lode and the School Section
triplites are to be made in the future. It is certain, however, that triplite,
can no longer be regarded as a simple series between Fe'' and Mn, as
indicated by Otto (1936) and Richmond (1940). In some triplites magnesium enters
into the composition in important quantities; and, as Hurlbut (1936, p. 656)
noted, the optical and other physical properties clearly reflect this variation.
ALTERATION
Triplite is commonly altered to black manganese oxide which coats the crystals
and stains the surrounding minerals. Most of the triplite crystals and pods also
are enclosed in a thin shell of altered material which lacks the usual vitreous
luster and is also somewhat darker in color. This outer skin has been variously
described as "pseudotriplite" or a "substance near heterosite"
(Dana, 1892).
The material of the outer shell has an nZ index of refraction considerably lower
than that of triplite (in the Mica Lode mineral, 1.600 as compared to 1.668),
but x-ray powder photographs of the shell and the fresh triplite core are
identical. The shell probably represents the initial stage in the breakdown of
the triplite. The isostructural decomposition product may be the result of
partial oxidation of the iron and manganese. Under the microscope the outer
material is nearly opaque owing to the presence of very abundant minute dark
brown inclusions.
NOTES
1 Contribution from the Department of Mineralogy and Petrography, Harvard
University, No. 286.
2 Boston University, Boston, Massachusetts.
3
University of Michigan, Ann Arbor, Michigan.
REFERENCES
1. BASTIN, E. S. (1911), Geology of the pegmatites and associated rocks of
Maine: U. S. Geol. Survey, Bull. 445, 18.
2. DANA, E. S. (1892), System of Mineralogy, 6th ed. New York, p. 778.
3. HEINRICH, E. Wm. (1947), Geology of the Eight Mile Park pegmatite area,
Colorado: Unpubl. Ph.D. thesis, Harvard University.
4. HESS, FRANK L., AND HUNT, W. F. (1913), Triplite from eastern Nevada: Am.
Jour. Sci., 36, 51-54.
5. HURLBUT, CORNELIUS S., JR. (1936), A new phosphate, bermanite, occurring with
triplite in Arizona: Am. Mineral., 21, 656-661.
6. KUNZ, GEORGE F. (1884), Topaz and associated minerals at Stoneham, Me.: Am.
Jour. Sci., 27, 212-216.
7. OTTO, HELMUT (1936), Die Rolle des Mangans in den Mineralien: Min. Mitt., 47,
89-140.
8. RICHMOND, WALLACE E. (1940), Crystal chemistry of the phosphates, arsenates
and vanadates of the type A2XO4(Z):
Am. Mineral., 25, 441-479.
9. SHANNON, EARL V. (1921), Notes on anglesite, anthophyllite, calcite, datolite,
sillimanite, stilpnomelane, tetrahedrite and triplite: Proc. U. S. Nat. Mus.,
58, 437-453.
10. SHEPARD, CHARLES W. (1852), On the triplite (alluandite?) of Norwich,
Massachusetts: Am. As. Pr., 6, 234-235.
Footer for links and copyright





