| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
Volume 9, pages 203-252, 1925 THE CAUSE OF COLOR IN SMOKY QUARTZ AND AMETHYST* EDWARD F. HOLDEN, University of Michigan ABSTRACT The nature of the pigments of smoky quartz and amethyst was investigated from the standpoints of the occurrence and genesis of those minerals, the effect of heat and of radiations upon the colors, the transmission of light, and analysis for the various impurities. It is concluded that amethyst owes its color to a ferric compound, while smoky quartz is probably pigmented by free atomic silicon, liberated through the action of radioactive substances. The literature is discussed as fully as space permits and a chronological bibliography is appended. I. INTRODUCTION THE SCOPE OF THIS INVESTIGATION The causes of the colors of smoky quartz and amethyst have been the subject of numerous investigations, in most of which only one or two methods of inquiry have been pursued. But, any attempt to discover the nature of the pigment of a dilute-colored mineral should be based upon as many different kinds of experimental evidence as possible. The opinion arrived at by a single method of investigation is not to be compared in plausibility to a conclusion substantiated by a number of converging lines of, evidence. Therefore the research here described considered the following principal subjects, all of which contribute their share to the final conclusions: 1. The occurrence and genesis of smoky quartz and amethyst. 2. The influence of radiations upon their colors 3. Color changes resulting from heat-treatment of these minerals. 4. The transmission of light through amethyst and smoky quartz. 5. The nature and amount of the impurities in these varieties of quartz. A large number of specimens were studied in order that some generalizations, applicable to all occurrences of these minerals, might be made from the results obtained. The portion of the study devoted to impurities is almost wholly new. Those parts dealing with the transmission of light, the effect of heat upon the colors, and the occurrence and genesis are largely original. However, very little new work on the effect of radiations was carried on, since that field has been very thoroughly covered by other investigators. HYPOTHESES CONCERNING THE PIGMENTS OF SMOKY QUARTZ AND AMETHYST Various investigators have suggested that smoky quartz is colored in one or another of the following manners: a) The pigment is an inorganic compound, probably of trivalent titanium. (15, 16).1 b) The pigment is a carbon compound. (7, 9, 18, 19, 28). c) The pigment is a suspension produced by the action of radium radiations. (37, 42). The ideas which have been advanced as to the cause of the color of amethyst are very similar, and may be classified as follows: a) The pigment is an inorganic compound. (1) It is a compound of iron. (2, 6, 11, 20, 22, 34, 56). (2) It is a compound of manganese. (28, 43). b) The pigment is a carbon compound. (18, 19). c) The color is due to the action of radium radiations. (41, 42). Each of the hypotheses as to the nature of the pigments is discussed in detail in later portions of this paper. LOCALITIES AND DEPTHS OF COLOR OF THE SPECIMENS STUDIED The localities and the depths of color of the various specimens of smoky quartz used in this investigation are given in Table I, below. The variation in color is indicated by four color-classes. Class i includes the dark blackish brown specimens, darker than Ridgway's 2 13"m; class ii, smoky brown, 17''' 13"m; iii, pale grayish brown, lighter than 17'''; and class iv, almost colorless. The order of arrangement within the four classes is not intended to be significant.TABLE 1. LOCALITIES AND DEPTHS OF COLOR FOR SMOKY QUARTZ
Table II gives the same data for the specimens of amethyst. Class i includes the dark violet specimens, corresponding to Ridgway's 63'm-63'; class ii, violet, 63'-63'b; iii, pale violet, 63'b-63'e; and class iv, very pale violet, 63'e-colorless. TABLE II. LOCALITIES AND DEPTHS OF COLOR FOR AMETHYST
1 Specimens 17a and 17b are dark and pale portions of the same group of crystals. ACKNOWLEDGEMENTS Grateful acknowledgement is made of the following assistance rendered during this investigation: The Department of Physics of the University of Michigan kindly permitted the use of a photospectrometer, and of an electroscope for some time, and several members of the staff gave freely of their time and advice. Dr. D. C. Bardwell, of the U. S. Bureau of Mines, radiated several sections of rock crystal. Considerable aid in obtaining specimens was given by Dr. H. V. Ellsworth, of the Canadian Geological Survey; Messrs. M. G. Biernbaum, of Philadelphia, Pennsylvania; W. J. Elwell, Danbury, Connecticut, and W. J. Paquette, Toledo, Ohio; the Philadelphia Academy of Natural Sciences and Ward's Natural Science Establishment, Rochester, New York. Dr. S. C. Lind, of the U. S. Bureau of Mines, and Professor Waldemar Lindgren, of the Massachusetts Institute of Technology, gave their advice and opinion with regard to certain problems encountered in the work. Professors E. H. Kraus and W. F. Hunt, of the Mineralogical Laboratory of the University of Michigan, kindly supervised the entire investigation and made many indispensable suggestions. II. PHYSICAL PROPERTIES OF SMOKY QUARTZ AND AMETHYST Only those properties in which smoky quartz and amethyst differ from ordinary quartz will be considered here. COLOR - The color of smoky quartz varies from a pale and somewhat yellowish brown (Ridgway's 19'''f), or a pale grayish brown (17''''f), through wood brown (17''') and dark brown (13''m) to black. Amethyst is more constant in hue, ranging from colorless to deep violet (about 63'g to 63'm). CRYSTAL FORM - Amethyst is nearly always well crystallized, and smoky quartz frequently so, in contrast to the universally massive form of rose quartz. 3 Smoky quartz and amethyst were crystallized slowly from aqueous solutions while rose quartz must have formed more quickly from a pasty aqueo-igneous fusion.The abundant occurrence of trigonal trapezohedrons and bipyramids upon smoky quartz crystals is testified to by numerous citations in the literature. Unequal development of r and z is also often noted. Amethyst, too, may show these characters. Smoky quartz, and more especially amethyst, are often twinned, the boundaries between the individuals in the twinned crystals being generally sharp and quite regular. Sections of smoky quartz or amethyst crystals are generally free from fractures. These characteristics unite in indicating that smoky quartz and amethyst were formed as the alpha or low-temperature form of quartz. (see part III). ZONAL COLORING - Both of these varieties of quartz are very often zonally colored. Indeed, amethyst almost always has a zonal distribution of color, which is of two types, frequently combined in a single crystal: 1. In fine lamellae parallel to the rhombohedron faces. 2. A type best revealed by a basal section, which shows a division into sectors. One half of these are violet, and all the other sectors, alternating with those that are violet, are either colorless, yellow, or smoky. The optical anomalies arising from the intimate twinning of the right and left lamellae and sectors, which correspond to the differently colored areas, are well described and illustrated by Tutton (51, 57). Intergrowths of smoky quartz and amethyst are frequent. DICHROISM - The absorption of smoky quartz is e>o, and the dichroic colors are as follows, depending upon the depth of color:
The pleochroism of rock crystal which had been colored brown by radium radiations was found to be identical with that of natural smoky quartz. In amethyst the dichroism is less uniform than in smoky quartz, due to the usual zoning of the colors. The specimens examined showed (1) reddish purple to purple, and (2) purple, bluish purple, or indigo. In some cases no dichroism was apparent. Haidinger (4) made an extensive study of the pleochroism of amethyst, and his results show the absorption to be e>o, with the color for e more reddish than that for o. INDICES OF REFRACTION - The variation of the indices of refraction with the color in quartz has been studied by Forster (7), Dufet (13), Hlawatsch (17), and Wülfing (30). The values which these investigators found for amethyst (ω 1.54418 4 to 1.54427) lie entirely within the range for rock crystal (ω 1.54418 to 1.54433). The index of an amethyst from Uruguay was increased 5 x 10-5, by heat-decolorization, according to Wülfing. Most of the measurements for smoky quartz agree closely with those of the other two varieties. In the fifteen determinations recorded for smoky quartz the value for ω was between 1.54403 and 1.54436 for thirteen specimens; in the other two cases ω was given as 1.54387 and 1.54388, respectively. Wülfing and Forster found that the heat-decolorization of dark smoky quartz caused no change in the index of refraction, but Hlawatsch noted a slight increase in the fourth decimal place Even the largest variation in refractivity from one specimen of smoky quartz to another is very slight, rarely attaining a magnitude of several units in the fourth decimal place.It is unlikely that the pigmenting impurities constitute any large proportion of the total impurities which effect the optical properties of these types of quartz. For this reason it is impossible to draw any plausible conclusions as to the nature of the pigments from this type of evidence. III. OCCURRENCE AND GENESIS OF SMOKY QUARTZ AND AMETHYST The production of the characteristic color of such minerals as smoky quartz and amethyst is due to the coexistence of the proper chemical and physical environment in the solutions from which they form. It is necessary that there be present those chemical compounds which constitute the pigment, and it is just as imperative that the mineral crystallizes under favorable physical conditions. Temperature is probably the most important of the physical factors. At this point in the study an effort will be made to determine the chemical and physical conditions prevailing during the formation of smoky quartz and amethyst. OCCURRENCE The occurrences of smoky quartz and amethyst may conveniently be classified into the six groups discussed below. With the exception of the last, they are arranged in the most probable order of decreasing temperature and pressure conditions. 1. IN CAVITIES IN DEEP-SEATED IGNEOUS ROCKS, PRINCIPALLY PEGMATITES - Both amethyst and smoky quartz occur frequently in the deep-seated igneous rocks, especially in the drusy cavities of pegmatites. These minerals crystallized out from hot aqueous solutions containing a large amount of carbon dioxide and other mineralizers. Quartz crystals generally coat the walls of the cavities and are among the last differentiates from the magma.Smoky quartz occurs in a wide variety of pegmatite types, comprising: (a) those with potash feldspar (e.g., Madagascar); (b) gem beryl pegmatites (Mourne Mountains, Ireland); (c) the Li-F-B type, with gem tourmaline and lepidolite (Mount Mica, Maine); (d) the Li-F-Mn-phosphate type (Branchville, Connecticut); (e) the cassiterite and tourmaline pegmatites (Fichtelgebirge); (f) the Cb-Ta-U-rare earth pegmatites, with radioactive minerals (many localities in Ontario). Smoky quartz is also found in the body of pegmatite veins, where it was often the last constituent to crystallize. Amethyst is less abundant in pegmatites than smoky quartz and it is not found in the body of the pegmatite veins, but occurs only in the cavities. The drusy cavities containing amethyst and smoky quartz are not confined to pegmatites, though they are most abundant there. Similar pockets often occur in granites, as well as in other deep-seated rocks. 2. IN HYDROTHERMAL VEINS CLOSELY ASSOCIATED WITH GRANITES AND PEGMATITES - These quartz veins were formed by the silica-bearing aqueous solutions which were the last differentiation products of acidic magmas. Amethyst and smoky quartz are sometimes found in cavities in such veins. 3. IN THE ALPINE TYPE OF VEINS - The Alpine veins have been thoroughly studied by Koenigsberger (24, 41, 44). They were formed by hot, ascending waters, rich in CO 2, which leached out the constituents of the rocks through which they passed. The dissolved substances later crystallized out in new combinations. Naturally, the composition of the minerals thus formed was determined by the nature of the leached rock.Smoky quartz is abundant in these veins, while amethyst is not infrequent. Smoky quartz is found in the veins in adularia gneiss, biotite gneiss, granites, and acid like rocks, but the quartz in the veins traversing schists of sedimentary origin and basic igneous rocks is almost always colorless. The most important associates of smoky quartz are as follows: (a) formed before or with the quartz: adularia; and (b) formed after the crystallization of the smoky quartz: fluorite characteristically red, calcite, the zeolites, and chlorite. Koenigsberger (24) states that in the central Alps the intensity of color of the smoky quartz crystals depends upon the altitude of the occurrence. He gives the following data for the western part of the protogene:
Brauns (37) thinks that the color may have been due to radium and that the radium may have been more active at higher levels, or that a lower temperature in the higher rocks permitted a more intense color to be produced by the radium. Most probably the explanation lies in a temperature effect. If the present altitude of the occurrences represents the proportional depth of the quartz when it was formed, the temperature of the veins now at 1400 m., would at that time have been 45° more than that of the veins now at 2900 m., assuming an added temperature of 1° for each increment of 33 m. in depth. This is a sufficient temperature range to allow the production of the different degrees of color observed (see part V). Amethyst in the Alps is always accompanied by iron minerals. Limonite is the most frequent associate, others being ankerite, siderite, and chlorite. 4. IN ORE VEINS - Colored quartz is not infrequently found in metalliferous veins. Amethyst occurs in such deposits more often than the smoky quartz. Amethyst has often been noted in the silver veins which Lindgren 5 describes as "deposits formed near the surface by ascending thermal waters and in genetic connection with igneous rocks." Examples are the deposits at Schemnitz, Hungary, and Guanajuato, Mexico. It is also noted in the ore-bodies classified as metalliferous deposits formed at intermediate depths, as, for example, in the lead-silver veins of Pribram, Bohemia, and in the silver veins on the north shore of Lake Superior. Such minerals as the carbonates, the sulfides, barite, and fluorite are common associates of amethyst in ore veins.When smoky quartz is found in ore veins it is generally in those which are mineralogically related to pegmatites, such as the cassiterite veins of Saxony. 5. IN THE AMYGDALOIDAL CAVITIES OF BASIC IGNEOUS ROCKS - Amethyst is very frequently found in the cavities of basic eruptive rocks. The associated minerals are agate and chalcedony, formed earlier than the amethyst; and datolite, prehnite, pectolite, apophyllite, the zeolites, and calcite, formed later. In some localities it seems evident that these minerals were precipitated from magmatic liquids in the gas cavities of cooling lavas; in others they were formed by the action of atmospheric waters percolating through recently erupted lava flows. Amethyst is found in veins and geodes in the Triassic traps of New Jersey, the Connecticut Valley, and Nova Scotia. Smoky quartz is only occasionally noted in these rocks. Another well known occurrence for amethyst is in the chalcedony and agate geodes from melaphyres in Brazil and Uruguay. C. RELATIVELY UNIMPORTANT MISCELLANEOUS OCCURRENCES A few occurrences of amethyst and smoky quartz in calcareous rocks, sandstones, and quartzites, in which there was no known genetic connection with igneous rocks, have been reported. In these instances the quartz crystals must have been deposited by waters of only moderate warmth. Amethyst often occurs in the agatized trees of Yellowstone Park and Arizona, the silicification having been caused by cool waters of meteoric origin. ELEMENTS ASSOCIATED WITH SMOKY QUARTZ AND AMETHYST Many other elements occur in the silica-bearing solutions from which these varieties of quartz crystallize. Minerals containing the following elements are frequently found with both smoky quartz and amethyst: H, as water; C, as CO 2Many of the rarer elements are more characteristically associated with smoky quartz than with amethyst. These include: the less common alkalies, Li, Rb, and Cs, in lepidolite, tourmaline, alkali beryl, and spodumene; Be, in beryl; P, in apatite and other phosphates; Sn, in cassiterite; W, in wolframite; Mo, in molybdenite; and in the numerous rare earth and radioactive minerals: Cb, Ta, Th, U, Ra, the rare earths, and Zr. The frequent occurrence of smoky quartz in association with rare earth and radioactive minerals is very significant, for later it will be indicated that smoky quartz may have been colored through the action of radioactive elements. In Ontario smoky quartz is a constant associate of radioactive minerals, 6 and the same association is frequent in Madagascar.7 Enormous smoky quartz crystals occur in the well known radioactive pegmatite of Baringer Hill, Texas.Only very pale varieties occur elsewhere than in acid igneous rocks, which are much more radioactive than other types of rocks. 8 There is, therefore, a correlation between the occurrence of smoky quartz and the radium content of the rocks in which it is found.Amethyst is often accompanied by minerals of some elements rarely found with smoky quartz: S, As, Cu, Zn, Pb, Ag, and Au, in the sulfides, sulfo-salts, and native metals; Ba, in barite; the compounds of Fe, limonite, goethite, hematite, and siderite, which are the most characteristic associates of amethyst, especially the darker varieties. One of these iron minerals invariably accompanies amethyst in the Alps (24, 41, 44). Amethyst is associated with limonite veins in Lincoln County, North Carolina. It occurs on siderite at Macskamezö in the Siebenbürgen, and on carnelian containing 3 per cent Fe 2O3 in the "Buntsandstein" of Waldshut, Baden. At Hüttenberg, Carinthia, it is found as druses on siderite and limonite. Near Onega-See, Russia, amethyst enclosing goethite needles is found.A specimen from El Paso County, Colorado, was zoned in smoky brown and violet. There were a. few scattered needles of goethite in the smoky area, but the violet portions are literally crowded with them. In the Lake Superior district much of the amethyst has inclusions of hematite or goethite. In specimens examined the color was deepest for several mm. below the thin layer of hematite inclusions, the rest of the crystal being white or colorless. These relations indicate that the violet quartz began to be formed when a sufficient concentration of iron was attained in the mineral solutions, the quartz previously formed being colorless. As the amount of iron increased the color became darker, and finally the deposition of hematite took place. In Madagascar (49) also, red and black hematite inclusions occur in amethyst. Many more instances of the occurrence of iron minerals with amethyst might be cited. It is significant that the amethyst from amygdules in basic igneous rocks is usually very dark, while that in pegmatites and related veins is most apt to be pale. Basic igneous rocks are high in iron; acid rocks are low. While many elements occur in the solutions from which amethyst has been deposited, iron is the only pigmenting substance which is characteristically present. The facts presented in the preceding paragraphs are good evidence that iron is essential in producing the color, a conclusion which data given later will substantiate. The chemical factors necessary in the formation of smoky quartz and amethyst, aside from the presence of the compounds causing the colors, are a moderate amount of uncombined silica in the aqueous solution, with considerable carbon dioxide and other mineralizers. With too great a concentration of silica the cryptocrystalline or poorly crystallized varieties of quartz are likely to be formed. At temperatures within the formation range of opal, the presence of carbon dioxide seems to favor the growth of quartz instead of opal. 9PARAGENESIS The paragenetic relationships of smoky quartz, amethyst, and the more important associated minerals are shown in Table III. Very little additional discussion is necessary. These varieties of quartz were generally formed after the colorless or white quartz of the pegmatites in which they occur, and after the chalcedony and agate of basic rocks. In the first instance, this is due to the temperature, which at first is too high to allow the pigmenting of the quartz. In the basic rocks, the cryptocrystalline varieties are at first precipitated from the concentrated silica solutions, to be followed later by the more slowly formed crystals. TABLE III. PARAGENESIS OF SMOKY QUARTZ AND AMETHYST
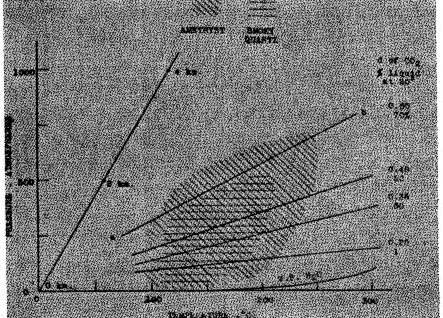
X indicates "generally" x indicates "occasionally" TEMPERATURE OF FORMATION The minimum temperatures at which decolorization occurs is shown in part V to be approximately 225° for smoky quartz and 260° for amethyst. It will be of interest to ascertain whether other lines of evidence unite in indicating a formation temperature below the point of decolorization. CRYSTALLOGRAPHIC EVIDENCE - Wright and Larsen 10 have indicated criteria which may be used to distinguish quartz formed above 575° from quartz formed below that temperature. As shown by the description of the physical properties in section II, the application of these criteria to smoky quartz and amethyst prove unquestionably that they were formed below 575°, as the alpha modification of quartz.EVIDENCE FROM LIQUID AND GASEOUS INCLUSIONS - Quite precise information concerning the temperature and pressure conditions during the formation of smoky quartz and amethyst is afforded by the abundant liquid-gas-filled cavities. From an extensive study of artificial and natural crystals, Sorby 11 concluded that "at the temperature at which they were formed, the fluid cavities in crystals are full of fluid, and . . . . at a lower temperature they contain vacuities, owing to the contraction of the fluid on cooling . . . . . The temperature (of formation) . . . . might be ascertained by determining what increase of heat would be required to expand the fluid so as to fill the cavities."Much more recently, Johnsen (47) by the application of accepted physico-chemical relations, has been able to construct a temperature-pressure curve, at some point along which an amethyst crystal studied by him must have been formed. This crystal contained a cavity filled with CO2. At 20° both liquid and gaseous phases of CO2 were present in the ratio of 70 to 30 by volume, respectively. At 30°, approximately the critical temperature of CO2, the whole inclusion became gaseous. From the volume ratios of the two phases at 20° and their known densities 12 Johnsen calculated the density of the originally included CO2 gas to be 0.60. Using van der Waal's equation, he then calculated the pressure-temperature curve along which carbon dioxide would have that specific gravity. (This is the curve ab of Figure 2 in this paper). The amethyst crystal in question must undoubtedly have formed at a temperature and pressure falling at some point on the curve, but one quantity must be known if the other is to be found.It appeared that both the temperature and pressure under which a specimen of quartz was formed could be approximately determined if the mineral contained both water and carbon dioxide inclusions. Examination of a series of crystals showed that such is not infrequently the case. These gaseous and liquid inclusions may be classified into three types, as follows: 1) INCLUSIONS CONSISTING OF WATER ALONE, OR OF AN AQUEOUS SOLUTION, BUT WITH NO FREE CARBON DIOXIDE - Such cavities contain small contraction bubbles, due to the cooling of the liquid from its temperature when enclosed in the growing quartz crystal The temperature at which the bubble just disappeared was determined. This is practically equal to the formation temperature, for the slight effect of pressure may be disregarded.
The fragment of quartz under examination was placed in a bath of melted paraffine, contained in a crystallizing dish on a microscope stage. The temperature of the bath, measured with a mercury thermometer, was gradually increased by means of a current passed through a small platinum resistance coil immersed in the liquid. The temperature at which a bubble in a cavity just vanished could thus be readily determined. In Fig. 1, sketches a, b, c, and d, made at room temperature, illustrate this first type of inclusion. In a and b, from smoky quartz No 14, the bubbles disappeared at 205±5°; in c and d, from amethyst No. 9, at 240± 10°. Negative crystal cavities are illustrated by b and d. 2) INCLUSIONS CONSISTING ENTIRELY OF CARBON DIOXIDE - Below the critical temperature, 31°, these frequently contain both the liquid and gaseous phases. Following Johnsen's method it is possible to construct a pressure-temperature curve, passing through the point at which the specimen must have formed. In Figure 2 are given the pressure-temperature curves for several densities of CO2. Sketches g and h, Figure 1, from fragments of amethyst Nos. 12 and 11, respectively, illustrate inclusions entirely of CO2. The inner bubble is gaseous, the outer zone liquid CO2. The sketches show the conditions at room temperature. Above 31° there is only one phase, gaseous CO2. 3) INCLUSIONS OF WATER WITH BUBBLES OF CARBON DIOXIDE AND WATER VAPOR - The CO2 in the cavity exceeds the amount soluble in water. Below 31° it may be either entirely gaseous CO2 or may exist as two phases. These CO2 bubbles in water furnish the same kind of information as is given by those inclusions entirely of CO2 The carbon dioxide bubbles can be distinguished from the contraction bubbles since the ratio of CO2 bubble to liquid is quite variable from one cavity to another in a single fragment, while in bubbles due solely to contraction the relation is necessarily quite constant. Undoubtedly, the CO2 in this third type must have been enclosed in the quartz as bubbles in the water. Bubbles of CO2 in water are illustrated by diagrams a and f (smoky quartz No. 15) and by i, a negative crystal cavity (smoky quartz No. 4). In the cavities represented here the density of the CO2 is such that it all remains gaseous at room temperature. When the first together with either the second or third types of inclusions occur in the same specimen, both the temperature and pressure at the time of formation are easily determinable. The water inclusions give evidence as to the temperature, which enables the pressure to be determined from the temperature-pressure curves afforded by the CO2 inclusions. The temperature determination is the more accurate because it involves no calculation. The cavities sketched in j and 1, Figure 1, at 25° are of the first and third types, respectively, and they occurred in the same crystal (smoky quartz No 5) The contraction bubble in j disappears at 135±°. Sketches k and l are of the same cavity at different temperatures. In k, the temperature is greater than 31°, when all the CO2 is gaseous. In l the innermost zone is a globule of liquid CO2 at the interface between the gaseous CO2 and the outermost zone of water. 13In the table given below are tabulated the results of examining a number of specimens of amethyst and smoky quartz in this way. TABLE IV. TEMPERATURE (t) AND PRESSURE. (p) OF FORMATION OF SMOKY QUARTZ AND AMETHYST AS DETERMINED FROM THEIR LIQUID AND GASEOUS INCLUSIONS
Figure 2 illustrates the paragenesis of smoky quartz, amethyst, and carbon dioxide. It is based on Johnsen's work, with. adaptations and much added data. The normal geothermobar, as shown, is that for a temperature increase of 1° per 33.3 m. increase in depth, and a pressure rise of 1 atm. per 4 m. The depths in the earth's crust are indicated at intervals of 2 km along this curve. The pressure-temperature curves for several densities of CO2 are given. To the right of these curves the corresponding proportion of liquid CO2 existing at 20° is indicated, in terms of its volume percentage of the whole inclusion of CO2. The vapor pressure-temperature curve for water is also given. The probable pressure-temperature conditions under which amethyst forms are represented by the area which is obliquely ruled, while the hypothetical region of formation of smoky quartz is shown by horizontal lines. It is obvious that amethyst is formed under a greater temperature and pressure range, both higher and lower, than smoky quartz, and that the two types may form simultaneously through a considerable variation in temperature and pressure, as frequently happens. The diagram (Fig. 2) shows that both of these varieties of quartz were formed under either lower pressure or higher temperature than normal for the earth's crust, or under both lower pressure and higher temperature. The latter is most probable. Those are the conditions to be expected in pockets containing hot aqueous solutions. The open cavities relieve their contents of the normal pressure, while the solution is hotter than the normal temperature because of its magmatic origin. The actual pressure must be that of the water vapor and gases. The pressure is shown by the inclusions to exceed that which would be caused by the water vapor alone at the several temperatures. EVIDENCE FROM PARAGENESIS - In respect to the formation temperature, the paragenetic relationships of these two colored varieties of quartz agree with the evidence from the inclusions. Adularia, formed before or with the earlier formed crystals of smoky quartz, is frequently deposited by waters of 50-150° in temperature, according to Lindgren. 14 Doelter15 concludes that orthoclase may have formed at temperatures as low as 100°. Both albite and orthoclase have been found in sedimentary rocks under circumstances positively demonstrating their formation after sedimentation.16 Muscovite, also of an earlier stage than smoky quartz, has been synthesized at 196-233°. Therefore, the lowest possible formation temperature for these earlier-crystallized minerals lies well below the highest possible temperature of formation for smoky quartz and amethyst, as indicated by the inclusions and the decolorization experiments.Calcite, fluorite, and chlorite, minerals of a later stage than smoky quartz and amethyst, are capable of formation through a rather wide range, down to very low temperatures. The zeolites are stable in a more restricted region and are characteristically low temperature minerals. They have been synthesized at temperatures as low as 100°, and in nature have been formed at even lower temperatures. Phillipsite, for example, is found in deep sea muds. The zeolites, fluorite, calcite, and chlorite could well have been formed below the lowest temperatures indicated for smoky quartz and amethyst. SUMMARY It is shown that amethyst and smoky quartz were crystallized from hot aqueous solutions in cavities, under less pressure than normal for the depth at which they were formed, and in a temperature range of about 110-220° for smoky quartz, and 90-250° for amethyst. The crystals grew rather slowly, from not too highly concentrated solutions. These solutions contained many other compounds than silica, notably carbon dioxide and other mineralizers. Smoky quartz is frequently accompanied by radioactive minerals. The iron minerals, hematite, limonite, goethite, and siderite are generally found with amethyst. IV. RADIATIONS AND THE COLOR OF SMOKY QUARTZ AND AMETHYST Much has been published concerning the effects of radiations on the color of smoky quartz and amethyst [Doelter (32, 33, 38, 39, 40, 42, 53), Egoroff (27), M. Berthelot (28), Miethe (29), D. Berthelot (31), Phillips (35), Simon (36), Brauns (36), Newberry and Lupton (45), Meyer and Pribram (50), and Lind and Bardwell (54)]. For the present investigation Dr. Bardwell kindly exposed three sections of New York rock crystal to the penetrating radiations from 230 mgs. Ra. for 112 days. The dichroism and absorption spectraum of these artificially colored specimens were found to be identical with that of the natural smoky quartz (see sections II and VI). The many investigations which have been reported by various workers allow very general conclusions to be made: a) The color of heat-decolorized amethyst and smoky quartz is restored by the penetrating radiations from radium. b) Pale specimens may have their colors intensified in the same way, but sometimes amethyst becomes brown on radiation. c) Colorless quartz normally becomes smoky brown, but sometimes is rather resistant to coloration by radium, and rarely becomes violet. d) A zonal coloring like that observed in naturally colored quartz crystals is sometimes produced by radiation. e) The radium-induced colors are unstable at moderate temperatures, and radiated specimens usually phosphoresce when heated. RELATION BETWEEN RADIUM RADIATIONS AND THE PIGMENTS OF SMOKY QUARTZ AND AMETHYST There is good evidence that the smoky brown color produced by the radiation of rock crystal is identical with the coloration of natural smoky quartz. The artificially colored variety agrees exactly with the natural in hue, dichroism, and absorption spectrum. Furthermore, the zonal coloring observed in natural crystals is often duplicated in the radiated rock crystal. Both artificial and natural smoky quartz are decolorized at moderate temperatures. These facts suggest that the pigmenting of natural smoky quartz is due to the radiation of colorless quartz by radioactive substances in the solutions from which it formed. This hypothesis will be further supported and elaborated. On the other hand, it does not seem possible to trace any inevitable connection between the action of radium and the natural production of the amethystine color. Radiation of almost any specimen of quartz, such as rock crystal, or rose quartz, or frequently even amethyst itself, brings about the brown coloration. On the contrary, the violet color is very rarely produced except when the color of pale or heated amethyst is deepened by radiation. The color of amethyst must be due to some characteristic pigmenting impurity, which other parts of the present study indicate to be a ferric compound. HYPOTHESES PROPOSED TO ACCOUNT FOR THE COLORATION OF MINERALS BY RADIATIONS The explanations which have been advanced to account for the production of color by the radiation of a mineral fall into two main classes: 1. The color is due to the formation of colloidal particles. 2. The color is due to the liberation of electrons without the production of colloidal particles. Doelter (40, 42), especially, has supported the first view. According to his view colorations are probably due to the formation of colloidal particles by the disintegration of impurities or of the pure mineral substance itself. The size and degree of dispersion of the resulting particles would determine the color produced, as in any other colloidal solution. Doelter suggests colloidal sodium or lithium, derived from included silicates, as the pigments in smoky quartz and amethyst. Decolorization by heating would be due to a change in the size of the particles or of their dispersity. Wild and Liesegang (52) have pointed out that it is very difficult to accept the hypothesis that solid colloidal particles might migrate through the rigid crystalline framework of a mineral. Lind and Bardwell (54) have recently proposed a theory which seems much more probable: Certain groups of electrons are thrown into metastable positions by the radiations. No displacement of the atom is involved, nor are any colloidal particles produced. Their assumption is that the displaced electrons are able to vibrate with a frequency which may fall in the visible region, causing the production of a color. They may return to their normal positions under the influence of heat. SCATTERING OF LIGHT BY SMOKY QUARTZ AND AMETHYST Strutt (46) has observed that a ray of light passing through a section of smoky quartz is strongly scattered, the path of the light being visible. In clear, colorless quartz, however, there was no scattering. Raman (48) has made similar observations. Vanzetti (55, 58) found the light-scattering in morion to be pronounced. After decolorization of the section at 300° the path of the light ray was no longer visible. In a zonally banded section light was scattered by the brown bands, but not by those which were colorless. Vanzetti concluded that it was plausible to suppose that the light-scattering and the color, both destroyed by beating and restored by radiation, were due to a colloidal suspension whose particles might vary in size. Perhaps a partial decomposition of the SiO2 was involved. Some observations made during the present study verify Vanzetti's experiments on smoky quartz The scattering of light from quartz was found to be of three types: 1. From microscopically visible cavities and inclusions. Common to all quartz, and unaffected by heat. 2. From long narrow areas of scattering particles. Noticeable in rock crystal as well as in smoky quartz. Neither of these first two types concern the color. 3. The third type of light-scattering has a connection with the color. It is impossible to detect any microscopically visible particles which could be responsible for this effect. There is a general scattering in the entire path of the beam. In the following paragraph only this type is considered. Eight specimens of smoky quartz were examined for this phenomenon. The effect was decidedly stronger in the darker specimens. From each of two specimens of smoky quartz (Nos. 6 and 8) pairs of sections were cut from single crystals. One section of each pair was decolorized by long and gentle heating, while the other was reserved for comparison. In both cases the original section scattered light strongly while the decolorized section showed little or no scattering. It is very evident, then, that the coloration of smoky quartz is to be correlated with the scattering of light by microscopically invisible particles, which were probably produced by the action of radioactive substances. It seems probable that the particles are of atomic rather than colloidal size. There are great difficulties to be met in explaining the migration, agglomeration, and dispersion of colloidal particles in a crystalline structure. As shown by evidence to be given later the pigmenting and light-scattering particles may well be atoms of elemental silicon. In seven amethyst specimens there was no light-scattering, which is further evidence opposed to coloration by colloidal alkalies, the theory which has been proposed by Doelter (40, 42). SUMMARY The evidence given thus far is in agreement with the theory that smoky quartz owes its color to atoms of silicon, formed by the disintegration of silica, through the action of radium radiations. The mechanism of the formation of the free silicon may perhaps be pictured in this way: The radiations may remove the four outer electrons from a silicon atom, which would then be equally shared by the two associated oxygen atoms. As a result, two free oxygen atoms and a free silicon atom would be formed. They could take no part in the crystal structure since their attractive force for other atoms would have been destroyed. Hence, they should act as small inclusions, the silicon atoms producing the light-scattering and the color so characteristic of smoky quartz. We would expect the silicon atoms to be most effective in scattering light because of their greater atomic weight, and the possibility of the escape of the oxygen atoms. V. THE COLOR CHANGES CAUSED BY HEATING Several investigators have made rather detailed studies of the heat-decolorization of smoky quartz and amethyst. The usual method of study has been to gradually increase the temperature, noting the points at which the various changes in color occur. But, with longer heating at a constant temperature, the decolorization takes place at a lower temperature than that given by the first method. Therefore it was thought desirable to more thoroughly investigate the influence of long-continued heating upon the decolorization. This new data, combined with the large number of observations reported by other investigators, affords, quite a complete knowledge of the effect of heat upon the colors of smoky quartz, and amethyst. REVIEW OF THE LITERATURE Simon (36) and Herman (34) have investigated the decolorization of these minerals in various reducing and oxidizing atmospheres. They found that the surrounding gas had no influence on the color changes in smoky quartz and amethyst. Simon worked with a large number of specimens and heated them both in hydrogen and oxygen. In. smoky quartz the first change was to a smoky- or greenish-gray. The mineral began to be decolorized at 300° and decolorization was complete at 330-370° in less than an hour. Heating for 48 hours at 290° also caused complete loss of color. With amethyst there were several distinct color changes as the temperature was increased. At 170-210° the specimens became gray violet. The change to colorless began at about 300° and was complete at 400 to 500°. A yellow coloration frequently superseded the colorless stage, and finally, above 700°, the specimens became milky white. Hermann (34) heated specimens of smoky quartz and amethyst at about 700° for two hours in the following atmospheres: air, oxygen, illuminating gas, sulfur vapor, hydrogen, nitrogen, ammonium chloride vapor, and ammonia gas. The color of all amethyst specimens changed from the original hue through gray-violet and yellow stages to opalescence. All the fragments of smoky quartz became colorless and clear. Wild and Liesegang (56) have recently investigated amethyst. All of the specimens became colorless by 500°, and on further heating became milky. The darker specimens often became smoky yellow, while the paler ones changed to clear yellow. One specimen, studied in detail and heated in air, began to be decolorized at 180-200°, was completely colorless at 340°, and became pale yellow at 350°. Less detailed work has been done by other investigators. Heintz (6) decolorized a specimen of dark amethyst at 250°. Berthelot (28) found the decolorization temperature of an amethyst to be 300°. The color of deep black morion was lost at 290°, according to Forster (7). Koenigsberger (21) found the decolorization tem perature of smoky quartz from several localities to be 295° after six to seven hours, and 370° after several minutes of heating. He also completely decolorized a specimen of smoky quartz in a bomb at 400° and 400 atmospheres pressure.NEW OBSERVATIONS In the measurements made at 235 ± 10°17 and at 240± 10° the fragments of quartz, from one to three cm. in diameter, were heated on an electric hot plate in a pyrex flask. A thermometer was inserted through the pierced cork, its bulb being placed beside the fragments. For the determinations at higher temperatures, a small electric oven was constructed. The temperatures were measured by means of a mercury thermometer. TABLE V. HEAT-DECOLORIZATION OF SMOKY QUARTZ Explanation of table. - At each different temperature (i.e. 235°, 275°, etc.) new specimens were taken. The colors are given in Ridgway's terms, and they are also designated by less exact but more readily understood terms. The colors were determined both when the specimens were hot and after they bad cooled to room temperature.
TABLE VI. HEAT-DECOLORIZATION OF AMETHYST
The decolorization of smoky quartz is plainly a time-temperature reaction. At 380-420° the decolorization is complete and immediate. Rapidly increasing time is required to discharge the color as the temperature of heating is lowered. Sometimes there is a tendency for the decolorization at low temperatures to be more or less incomplete, as in the case of specimen No. 6. The almost black original color is completely discharged at 420°, but at lower temperatures, no matter how long the heating is continued, an increasingly greater residue of the color remains. After being heated for eighty-one hours at 235±10°, the color was a medium brown, which did not change after a further exposure of sixty-nine hours at the same temperature, but the paler specimen No. 8 was brought to practically complete decolorization at that temperature. The size of the fragments is also a factor in the decolorization of smoky quartz. The following table shows the time necessary for complete decolorization of smoky quartz at various temperatures, or for attaining a pale but stable color. TABLE VII. TIME NECESSARY FOR DECOLORIZING SMOKY QUARTZ AT DIFFERENT TEMPERATURES
If a curve is plotted from these concordant data, time being the abscissae, temperature the ordinates, it will be of the parabolic type. The curve is very steep between 300 and 400°, finally merging into the vertical axis, where time = 0. At lower temperatures the curve rapidly flattens out until it is practically horizontal at 225°, which may be taken as the minimum decolorization temperature of smoky quartz, and the maximum temperature at which smoky quartz can have existed in nature (see section III). Tests on sixteen specimens of smoky quartz, from all four of the color classes, showed that in every case the color disappeared after heating for shorter or longer periods at 240± 10°. The results are given in Table VIII. It is evident that there is a general tendency for the darker specimens to require a longer time for decolorization than the lighter ones. TABLE VIII. TIME NECESSARY FOR DECOLORIZATION OF SMOKY QUARTZ AT 240± 10°
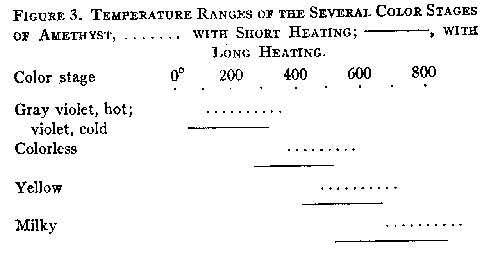
When hot, smoky quartz has a pronounced yellowish-greenish to blackish-greenish color. If the heating is not prolonged until decolorization ensues, the original color is regained on cooling. The decolorization of smoky quartz by heat can be explained as being due to the oxidation of silicon atoms, causing them to revert to their original character as parts of the quartz lattice. The decolorization of amethyst takes place at a higher temperature than that of smoky quartz. After eighty-two hours at 235°, only one of the three specimens tested showed any appreciable change of color, and it lost only a portion of its original color. After forty-nine hours at 305-315° the other two specimens were partially decolorized, but several hours heating at 385-420° were necessary to completely remove the color. This agrees with the results of other investigators. For most specimens of amethyst the minimum temperature at which the color is unstable may be given as 260±°. At lower temperatures, amethyst is always a gray violet when hot, though it again takes on its original color when cooled to room temperature. Further heating after the colorless stage has been reached often produces a citrine yellow color, especially in the darker specimens, as is well known. This is supplanted by an opaque milkiness at still higher temperatures. The diagram below shows the approximate temperature ranges of the various color stages.
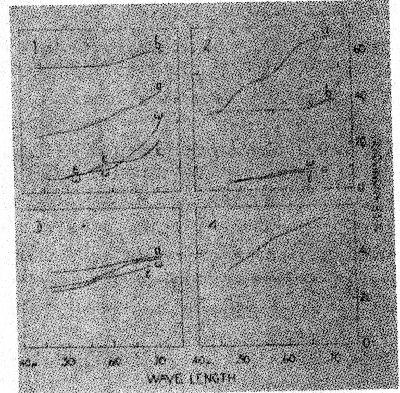
The change from violet to yellow, on heating, may be interpreted as due to the disintegration of a violet ferric compound to a simpler, yellow, ferric compound, possibly the oxide. This is further discussed in part VI. The changes in absorption spectra caused by heating smoky quartz and amethyst are described in the next section. VI. THE TRANSMISSION OF LIGHT BY SMOKY QUARTZ AND AMETHYST Frequently the manner in which a mineral transmits light will give a clue to the chemical nature of the pigment. For this reason the transmission of light through several specimens of smoky quartz and amethyst was measured. Heat decolorized specimens were also studied in the same way, as well as a section of rock crystal which had been colored by radium radiation. REVIEW OF THE LITERATURE Nabl (20) compared the spectra of amethyst, "burnt amethyst," which had been changed to yellow by heat treatment, and citrine. The amethyst possessed an absorption maximum in the green. After its color had been changed to yellow by heat, the absorption spectrum was identical with that of natural citrine. Nabl concluded that the spectrum of amethyst is identical with that of ferric sulfocyanate, and advanced the hypothesis that the violet color is due to that compound. However, neither the character of the absorption nor the color of ferric sulfocyanate solutions agree with those of amethyst. The maximum of absorption of ferric sulfocyanate in amyl alcohol is at 0.516μ18, while in amethyst it is at 0.53-54μ. The color of sulfocyanate solutions is an almost pure red (Ridgway 72) while that of amethyst is violet (63'). Added to these objections is the consideration that compounds of this nature have not been found to exist among minerals. Vanzetti (55) found that the maximum of absorption of light by smoky quartz is in the violet portion of the spectrum. NEW OBSERVATIONS The measurements here reported were made in the Physics Laboratory of the. University of Michigan. The instrument used was a photospectrometer with a variable sector disk. Polished sections, or crystals with smooth faces, were employed in this work. The results are graphically shown in Figures 4 and 5. The abscissae represent the wave lengths of the transmitted light in g, the ordinates, the percentage of the incident light which was transmitted through the sections. In Figure 4, Diagram 1, are given several transmission curves for a single section of smoky quartz. Curve la gives the transmission of ordinary light passing through the section in a direction parallel to the vertical axis. There is a gradual and steady increase in the percentage of incident light transmitted as the wave length increases. The curves marked w and a were obtained by passing plane polarized light through the section in a direction perpendicular to the c axis. These curves give the transmission for the ordinary and extraordinary rays, respectively. The somewhat yellowish cast of the color for a is due to the convexity of the curve near 0.60A. The curves also demonstrate that the absorption is e>w, as stated previously (part II). In these and later curves, too much attention must not be paid to the absolute amount of transmitted light. The more important feature is the shape of the curve, for the percentage of transmitted light varies with the thickness and clearness of the section.
The section, the transmissibility of which is given in curve 1a, was heated at 235±10° for twenty-two hours, which caused decolorization. New measurements, plotted in curve 1b, were then made. The direction of the passage of light is the same as in curve la, so that the two curves are comparable. As would be expected, the heat treatment markedly increased the transmission of light, and the transmission is about the same for all parts of the spectrum except that it is somewhat greater in the red. This is because decolorization was not quite complete. In Figure 4, Diagram 2, are given curves for three more specimens of smoky quartz. Curve 2a is similar to la. The specimen represented by 2b was very pale, and therefore its transmissibility is like that of heat-decolorized smoky quartz, 1b. Curve 2c shows the transmission of ca and a in another section. The transmissibility of rock crystal which has been colored smoky brown by radiation (diagram 3) is exactly like that of natural smoky quartz, as a comparison of diagrams 3 and 1 shows. Even the convexity in the curve for e, near 0.60μ, is found in the radiated quartz. Since it has been suggested19 that smoky quartz may owe its color to dispersoid silicon, the transmissibility of a solution of colloidal silicon was measured. This solution was prepared by producing an electric arc between two bits of silicon held under isobutyl alcohol. The curve for the colloidal silicon solution (curve 4) is very similar to that for smoky quartz, apparently making it possible that the pigment actually is colloidal or dispersoid silicon. Not too much stress should be laid on this, however, for it must be pointed out that many other elements give the same brown color when in colloidal solutions prepared by the electric-arc-dispersion method. In Figure 5, curves 1d and 1b, 2a, and 3a, show the transmission of light through amethyst. The curves are alike and show that amethyst has a maximum of absorption near 0.53-.54μ, in the yellow-green. This color, being complementary to violet, is responsible for the hue of amethyst. Curves 3a and 3b show the effect of heat treatment on amethyst. Curve a is for the original violet section. After being heated for one-half hour at 400° it became yellow, giving the transmission plotted in b. The latter curve is similar to those of citrine, 4a and 4b. These observations agree with those of Nabl (20), therefore, in showing that "burnt amethyst" and citrine have the same pigment, which is probably some simple compound of ferric iron. The transmissibility of a colloidal hydrous ferric oxide solution was measured (curve 4c) and found to be entirely similar to that of citrine. The violet pigment of amethyst is, then, probably a ferric compound, which is decomposed by heat to the yellow citrine pigment. The fact that the paler amethyst specimens do not give the yellow color on heating is probably due to a greater pigmenting power of the violet compound than the yellow one. The intermediate colorless stage may be due to a chromatic neutralization of the nearly complementary yellow and violet colorations.
While most ferric compounds are yellow or red in color, they frequently show a distinct, though slight, absorption maximum near 0.52°μ.20 This is the region in which FeSCN absorbs strongly. In view of the well known variations in the position and intensity of absorption by an ion depending upon its chemical environment, it may be said that the fact that amethyst has an absorption maximum at 0.53μ is in agreement with the hypothesis that it has a ferric pigment. Some ferric compounds have the same color as amethyst. It has been definitely proven that the violet ferric ammonium alum owes its color not to any impurity, but to the iron itself.21 If the alum is crystallized from an excess of sulfuric acid to prevent the formation of hydrous ferric oxide by hydrolysis, the crystals are violet. Their color exactly matches the hue of amethyst. Curves 2a and 2b of Figure 5 are of interest in showing the transmissibility of a violet area (curve a) and a smoky brown area (b) in the same zonally colored crystal. The curves are like those of uniformly colored crystals of the same hues, respectively. VII. THE IMPURITIES IN SMOKY QUARTZ Besides the theory that smoky quartz has been colored by the action of radium radiations, it has been suggested that its color may be due to trivalent titanium or to hydrocarbon compounds. The results of analytical work on the mineral will be given in this section, with a discussion of their application to all the theories that have been proposed to account for the color. ANALYSES OF SMOKY QUARTZ A detailed analysis was made on a twenty-five-gram sample of the dark colored smoky quartz No. 1, in order to discover what possible pigmenting elements might be present. The finely ground quartz was decomposed by means of hydrofluoric and sulfuric acids. The results of the analysis appear in Table IX, with a similar analysis for amethyst No. 1. The elements which might cause a coloration, and which were found by this analysis, are iron, titanium, and manganese. Therefore these impurities were determined on seventeen specimens of all depths of color (Table X). The method of analysis was as follows: Samples of finely ground quartz, of five to six grams weight, were decomposed in platinum dishes by means of HF and H2SO4 The sulfuric acid solution finally obtained, diluted with water, was first analyzed for TiO2 by the peroxide colorimetric method. The yellow coloration was then discharged by reduction with SO2 water, and the excess of SO2 was boiled off. Manganese was next determined colorimetrically by the KIO4 method. The periodate was then reduced with SO2, and the liberated iodine was boiled off. The solution was evaporated until fumes of SO3 appeared and, after dilution, Fe2O3 was determined by titration with N/200 KMnO4. TABLE IX. DETAILED ANALYSIS OF SMOKY QUARTZ NO. 1 AND AMETHYST NO. 1
In the analyses great care was taken to avoid introducing impurities through the reagents, all of which were analyzed for iron, titanium, and manganese. Only iron was found in any of them, and that only in small amounts. Proper corrections, never exceeding 0.4 mg. Fe2O3, were made on the final determinations. HYPOTHESES CONCERNING THE PIGMENT The several theories which have been advanced in explanation of the cause of color in smoky quartz have been outlined in the introduction. In section IV evidence was given favoring a coloration through the action of radioactive substances. It is now necessary, first, to examine the evidence for and against the other theories, and second, to present experimental results further substantiating the radiation hypothesis. TABLE X. ANALYSES OF SMOKY QUARTZ FOR Fe2O3, TiO2, AND MnO
1 "none"=less than 0.001%o
Fe2O3, the smallest amount detectable
CARBON COMPOUNDS - A number of investigators have concluded that smoky quartz owes its color to the presence of hydrocarbon pigments. Forster (7) and Wright (9) obtained extremely small amounts of oily liquids by heating large samples of smoky quartz fragments. This led them to think that the color was due to hydrocarbons. Berthelot (28) thought the pigment was a carbon compound because smoky quartz is decolorized by heating, with an "organic smell." Kraatz-Koschlau and Wöhler (18, 91) also advanced the hypothesis that smoky quartz was colored by a hydrocarbon, on the basis of such criteria as (a) decolorization simultaneously with the production of a "burnt smell"; (b) pyrophosphorescence; (c) the browning of the powder on being heated with concentrated H2SO4; (d) the determination of CO2 and H2O on igniting the ground mineral in an oxygen current. Several investigators have found very pertinent reasons for disbelieving that these features indicate the presence of a hydrocarbon pigment. Weinschenk (15) has observed that colorless quartz occurs in sedimentary rocks full of carbonaceous substances, while colored quartz is found in pegmatites, where hydrocarbon pigments could not have existed. Later (23) he replied to Kraatz-Koschlau and Wöhler, saying that the "odor of organic matter," "browning of the powder," and pyrophosphorescence are as easily produced on powdered glass as with a colored mineral. It is entirely unnecessary to heat quartz powder in an oxygen stream to obtain CO2 and H2O as they are practically always present in the original specimens as liquid and gaseous inclusions. It is not permissible to recalculate the CO2 and H2O found in this way, to C and H, and to assume the presence of hydrocarbons on that evidence. In this investigation a large sample of dark smoky quartz was analyzed to determine whether it would yield any colored hydrocarbon liquid. A forty-two-gram sample of specimen No. 4 was ground after careful washing with concentrated nitric acid, water, alcohol, and ether. The powder was soaked successively in alcohol, ether, and chloroform, for from seven to nineteen hours in each case. The decant liquids were evaporated to a smaller volume than that of the quartz sample, but they remained colorless. The powder was then heated to decolorization in a retort, but only a colorless liquid, neutral to litmus, was obtained. This was undoubtedly water. The results, then, were entirely negative. The previous determinations, by other investigators, of oily brown liquids obtained by distillation from smoky quartz may probably be ascribed either to imperfect cleaning of the specimens, or to the presence of inclusions in the quartz. Quartz has been known to contain cavities filled with petroleum or asphalt.22 TITANIUM - Weinschenk (15,16) ascribed the color of smoky quartz to T i2O3 in dilute solid solution, because he had obtained a positive test for titanium in several specimens in which he detected no rutile. The analyses given in Table X shows that the small amount of titanium found in smoky quartz is quite uniform from the darker to the paler specimens. If the color were due to that element, the darker specimens should contain decidedly more than those which were pale. Furthermore, amethyst contains approximately as much titanium as smoky quartz (Table XIII) and rose quartz considerably more.23 An examination of the specimens of smoky quartz showed in many cases minute needle-like inclusions, which are believed to be rutile, accounting for the presence of titanium in smoky quartz. It is therefore concluded that this variety of quartz does not owe its color to titanium. IRON AND MANGANESE - As in the case of titanium, the low percentages of iron and manganese occurring in smoky quartz have no apparent relation to the color. They have never been suggested as possible pigments for this mineral, as far as could be ascertained. URANIUM - It has been shown that none of the pigmenting elements found in smoky quartz can account for its color. Earlier in this paper the theory that smoky quartz has been colored by the action of radiations has been supported and elaborated. It is therefore of considerable interest to determine whether smoky quartz contains appreciable amounts of radioactive elements. Large composite samples (twenty-four to thirty-four grams) were employed in determining uranium. There were two samples for color class i, the darkest specimens. One was of specimens from radioactive pegmatites of Ontario. The other was a composite of specimens which did not occur in notably radioactive rock bodies, as far as known. The separation of uranium was carried out by Scholl's method.24 When all interfering elements had been removed, a little AlCl3 solution was added to aid in carrying down the uranium hydroxide. Sodium phosphate beads of the ignited precipitate were compared with standard uranium beads. The results are given in Table XI. TABLE XI. ESTIMATED PERCENTAGES OF UO3 IN SMOKY QUARTZ
The ignited precipitate of sample I was a decided green. The amount of UO3 indicated by these analyses is about fifty times greater than that ordinarily found in colorless quartz. Strutt25 has determined, in four specimens of quartz, an amount equal to 2 to 13 x 10-5 grams UO3 per 100 grams quartz. It was not possible to detect any uranium in amethyst (Table XIII) or rose quartz.26 In view of the small amount of disintegration necessary to produce a color when a mineral is radiated, the percentages of UO3 in smoky quartz are significant. This is especially so since the determinations probably give only a minimum percentage, due to the difficulty of completely separating traces of elements. The amount of UO3 is greater in the darker specimens, and less in the lighter. It is of interest to note that sample I contained more uranium than sample II, of the same color class, though the latter is from strongly radioactive rocks. In the Ontario occurrence the near presence of active material outside the quartz could easily have colored it. In order to determine radium, a large, composite sample of 102 grams from specimens Nos. 1, 4, 18, and 31 was prepared. The entire residue, after the silica was driven off with HF and H2SO4, was washed into a flask, which was sealed so that the radium emanation might accumulate. The sample showed decided activity when tested in an emanation electroscope. Approximately 4 x 10-10 grams Ra per 100 grams of quartz were found to be present. This is equivalent to 0.001% UO3, which is of the order of the amount found by direct analysis. The two methods therefore check in showing that smoky quartz contains many times more radium and uranium than is ordinarily found in quartz. Given the necessarily great time element, such amounts of radium could well cause the color of smoky quartz. The possible presence of thorium, which was not tested for, would increase the strength of the radiations. FREE SILICON - Having found support for the radiation theory of coloration in the presence of radium and uranium, it became necessary to test the further assumption that the coloration was due to the liberation of free silicon by the radiations. It is very difficult to determine the presence of traces of elemental silicon, but a method was devised which gives results that may, perhaps, be regarded as fairly good evidence of the presence or absence of free silicon. The principle of the method is that amorphous silicon is attacked and oxidized by aqua regia If this occurs, it would be expected that the newly produced silica would form as the colloidal hydroxide, which might be filtered off and recovered by the evaporation of the filtrate. Certain precautions as to blanks must be taken. The quartz was finely ground and was treated for a known period with boiling aqua regia of definite concentration. The acid was quickly diluted with water and was filtered through two quantitative filter papers. After the addition of sulfuric acid, the filtrate was evaporated until SO3 fumes appeared. The beaker was then carefully washed out into a weighed platinum dish. Evaporation to dryness was followed by ignition. After the residue was weighed, the silica was volatilized with HF, and the residue again weighed. The difference between these weighings gave the amount of silica recovered by this process. Blanks were run with definite weights of rock crystal powder, using the same amount and concentration of acid as in the smoky quartz tests. The time was also the same. This determined what amount of the finely ground quartz would be carried into colloidal solution when treated with hot aqua regia. All of the silica recovered from the smoky quartz in excess of the blanks was assumed to be derived from free silicon. In this way a minimum determination of the amount of free silicon in the quartz was obtained. The proportion of the total amount present that is recovered, of course, depends largely upon the fineness of grinding. In order to keep this factor as uniform as possible, large samples were ground, and each sample was divided into two parts. One was decolorized by gentle heating while the other was untreated. The amount of silica recovered from both the original and the heat-decolorized specimens was thus determined. In the same way, a sample of rose quartz which had been radiated until it became smoky brown was divided and one half was decolorized. A sample of unradiated rose quartz was analyzed in the same manner as those which had been radiated. The results of these analyses are given in Table XII. TABLE XII. AMOUNT OF SILICA RECOVERED FROM SMOKY QUARTZ, ROCK CRYSTAL, AND RADIATED ROSE QUARTZ
1 Average of three determination. The decolorized smoky quartz and decolorized radiated rose quartz gave about the same amount of silica as the rock crystal blanks. This is interpreted to be colloidal silica taken up by the action of the acid, rather than oxidized free silicon, but the original smoky quartz and radiated rose quartz specimens show significant excesses of recovered silica over the blanks and heated specimens. This appears to be an indication that they contain free silicon in appreciable amounts It is to be noted that the darker specimens of smoky quartz contain more free silicon than the paler, which is to be expected if silicon is the coloring agent. The figures given in Table XII represent the minimum amount of recoverable silica derived from free silicon. It would probably be quite safe to place the actual percentage of silicon in the darker smoky quartz specimens as of the order of 0.01 per cent. There are possible objections to the conclusion that these experiments indicate the presence of free silicon in smoky quartz and radiated quartz. It might be argued that the silica recovered by the process is obtained from an included hydrated alkaline silicate of a nature similar to that of the zeolites. Heating that type of compound would cause partial dehydration, and then it would less readily yield silica; but such an assumption does not explain why radiated rose quartz gives up considerably more silica than the unradiated type, and still more than the rock crystal. We are forced to conclude that there is a fairly strong indication of the presence of free silicon in smoky quartz, even though the evidence cannot be positive, due to analytical difficulties. SUMMARY It is probable that smoky quartz contains free silicon, which was liberated by radiation, and is responsible for the color of that mineral. This cannot be certainly and unquestionable proven, but the evidence given above indicates such to be the case. The presence of significant amounts of uranium and radium is also very important, for it adds to the evidence for coloration by radiation. No evidence for the presence of hydrocarbon pigments or of coloration by trivalent titanium was obtained. VIII. THE IMPURITIES PRESENT IN AMETHYST The results of the analyses of amethyst are given in this section, together with a discussion of the hypotheses that amethyst is colored by (a) hydrocarbons, (b) by a compound of manganese, and (c) by an iron compound. ANALYSES OF AMETHYST In order to determine what impurities were found in amethyst, a detailed analysis was made on nineteen grams of specimen No. 1, which was very dark colored. The results have been given in Table X, in part VII. The elements which might possibly enter into the pigment are iron, manganese, and titanium. Analyses for these impurities were therefore made on seventeen specimens, as shown in Table XIII. The methods of analysis were similar to those employed for smoky quartz, except that iron was determined both by titration and colorimetrically, by the improved HSCN method.27 The results of the Fe2O3 analyses by the two methods agreed very well, though for the amounts of iron encountered the titration method was the more accurate. TABLE XIII. ANALYSES OF AMETHYST FOR Fe2O3, TiO2, AND MnO
1 "trace" =less than 0.001% TiO2 In part I the various theories proposed to account for the color of amethyst have been outlined. The hypothesis of coloration by radium radiation is discussed in part IV, where it has been found to be inapplicable. CARBON COMPOUNDS - Kraatz-Koschlau and Wöhler (18, 19) have suggested that amethyst, as well as smoky quartz, is colored by a hydrocarbon, though possibly manganese has some connection with the amethyst pigment. Specimens from Brazil and Schemnitz obeyed all the criteria which they had given as indicating a hydrocarbon pigment (see part VII). Simon (36) detected traces of CO2 and H2O in amethyst, but he found more in colorless rock crystal. Other writers, in supporting their own theories, have disputed the possibility of a hydrocarbon pigment in amethyst [Doelter (42) and Heintz (6)]. MANGANESE - The theory that manganese compounds color amethyst was proposed by Berthelot (28). He treated quartz by radium radiation and decided that the violet color was due to a trivalent manganese compound, largely from analogy with the behavior of glasses containing a trace of manganese. There are several valid objections to this hypothesis. In the first place, the cause of the violet coloration of glass by radium is not definitely known, and it is the subject of dispute among authorities.28 It is not generally accepted that the color is due to manganese. Small amounts of manganese impart a pink, not a violet, color to glasses. Furthermore, rose quartz, the color of which is probably due to manganese,29 is pink, and its color is not deepened by radium radiations as is that of amethyst. Instead, both the original and heat-decolorized specimens of rose quartz always become smoky brown when radiated. Watson and Beard (43), too, believed that amethyst has a manganese pigment. This conclusion was based on four analyses which are in general agreement with those of Table XIII. It is to be noted that they regarded the coloration of rose quartz as due to an organic compound, although their analyses showed that it had on the average about as much manganese as amethyst. The high content of iron in amethyst was disregarded. The analyses in Table XIII show that the amount of manganese in amethyst does not vary with the depth of color. With the exception of specimens Nos. 19, 13, 17a, and 17b, the amount of MnO is very small. Specimens Nos 19, 17a, and 17b differ from the others analyzed in being massive specimens from ore veins, which accounts for the relatively high MnO content. The amount of manganese in No. 17b, a pale portion of the same specimen from which No. 17a was taken, is more than in No. 17a, the darker colored part. Specimen No. 13 contains canals filled with black manganese dioxide, easily visible to the eye. It is concluded, therefore, that manganese does not color amethyst. TITANIUM - The amount of titanium is small and rather uniform from the dark to the pale specimens. It is probably due to the small rutile inclusions often seen in quartz. IRON - The suggestion that an iron compound is the pigment of amethyst has been the one most often made. Poggendorff (2) suggested that it might be colored by an iron acid. Heintz (6) analyzed a specimen from Brazil and found 0.020 per cent Fe2O3 and less than 0.01 per cent MnO. He, also, believed that an iron acid might be the pigment. The change of color on heating would be due to the formation of a yellow oxide. Engler and Knies (11) proposed a ferric carbonate as the pigment. Hermann (34) thought the color might be due to the presence of ferrous, ferric, and manganese compounds. The action of heat, producing a yellow color, might be due to the loss of the violet manganese color, allowing the yellow iron color to predominate. Nabl (20) has advanced the belief that FeSCN is the pigment of amethyst. Objections to his conclusions, drawn from the absorption spectrum, are given in part VI. His analytical results will now be discussed. Nabl found sulfur in a fusion of amethyst powder with soda and niter. Kraatz-Koschlau and Wohler (19) tried to verify this determination, and ignited the quartz powder in a stream of oxygen which was led through sodium hypobromite. The solution was tested for sulfur with negative results. Nabl (22) then repeated his analyses, using new fusion methods, and again obtained positive tests. The apparent discrepancy between Nabl and Kraatz-Koschlau and Wohler's results is easily accounted for. The presence of sulfates in liquid inclusions in quartz has been noted several times.30 Nabl's methods, involving fusion, recovered the sulfur from the sulfates, but the other investigators, who simply ignited the quartz powder, could not break down the sulfates, though a sulfocyanate would readily have yielded its sulfur. Their results simply show that no FeSCN was present. Wild and Liesgang (56), using the spectroscopic method, found traces of iron in amethyst. They ascribed the color to that element. The analyses in Table XIII show that amethyst contains large amounts of iron, and that the percentage of Fe2O3 decreases quite regularly as the specimens become paler in color. The average percentage of Fe2O3 for each of the four color-classes is: i, 0.14; ii, 0.026; iii, 0.007; iv, 0.007 This seems clearly to show that some compound of iron is the pigment of amethyst. That view is supported by other evidence already given. All of the specimens were examined microscopically. Besides the generally present water and carbon dioxide inclusions, needles of goethite and particles of hematite were frequently found. These inclusions of iron compounds are most abundant in the darker colored specimens, and are not present at all in the paler ones. In Table XIII analyses Nos. 1, 3, 4, 14, and 19 are those in which part of the iron was contributed by these visible inclusions, which it was not possible to eliminate. The other specimens chosen for analysis are free of such particles. Endeavoring to separate the visible hematite inclusions from the iron compounds of submicroscopic dimensions, which must form the pigment, the powder of specimens Nos. 3 and 4 was leached with HCl, before the usual treatment with HF. In both cases the iron was practically all dissolved by the HCl, as shown in Table XIV An absolutely clear fragment of No. 7, which showed no inclusions under the microscope, was then analyzed in the same way. Again, all the iron was taken up by the HCl, showing that the visible hematite or goethite cannot be separated from the remainder of the iron compounds in this manner, for the ferriferous pigment is as completely soluble in HCl as is hematite. But, the very fact that iron oxide inclusions are most abundant in the darker specimens of amethyst, and are entirely absent in those which are pale, is evidence in favor of an iron compound as the pigment Even if analyses Nos. 1, 3, 4, 14, and 19 were omitted, the variation of iron content with depth of color is still very evident. TABLE XIV. SOLUBILITY OF THE IRON COMPOUNDS IN AMETHYST
IX. SUMMARY AND CONCLUSIONS SMOKY QUARTZ Smoky quartz occurs most typically in the drusy cavities of pegmatites and the similar Alpine type of veins. It is formed in the temperature range of about 101° to 220° C., crystallizing from solutions containing carbon dioxide and other mineralizers. The heat-decolorization of smoky quartz is a time-temperature reaction. Decolorization is complete and immediate at 400°, but continued heating at 235° will cause practically complete loss of color. When hot, the mineral is yellowish to blackish-green, but if heating was not too prolonged, it regains its original color on cooling. The light transmitted through smoky quartz is strongest in the red and steadily decreases to the violet end of the spectrum. The absorption is e>o with a slightly more yellowish than o. The amount of iron, titanium, and manganese in the mineral is small, and bears no relation to the depth of color. The pigment cannot be a hydrocarbon. It seems probable that smoky quartz owes its color and the property of scattering light to particles of free silicon, of atomic, not colloidal, dimensions. It is believed that small amounts of that element are liberated by the radiations from radioactive substances present in the quartz or in the solutions from which it crystallized. The principal points of evidence in favor of this hypothesis may be summarized as follows: a) Smoky quartz is characteristically associated with radioactive minerals. b) Nearly all quartz becomes smoky brown when radiated with radium compounds. c) The color of heat-decolorized smoky quartz is restored by radiation, and the color of pale specimens may be deepened in that manner. d) The dichroism and the absorption spectrum of smoky quartz agree exactly with those of radiated rock crystal which has become smoky brown. e) Smoky quartz strongly scatters light, and this property seems to be correlated with the coloration, for the dark specimens scatter light more strongly than the pale ones, and the scattering disappears with the color when the specimens are heated. f) The smoky quartz specimens examined in this study contained appreciable amounts of uranium, with radium in equilibrium. g) Experiments have indicated the probable presence, in smoky quartz, of free silicon, which is oxidized by heat, causing decolorization. Both uranium and silicon are most abundant in the darker specimens. The hypothesis which has been presented and supported above seems to fit the experimental evidence quite well. Originality for the idea here presented is not, of course, claimed, but additional evidence has been submitted and the theory modified so as better to satisfy the experimental results. AMETHYST Amethyst is characteristically zonally colored. It is generally found in amygdaloidal cavities of basic igneous rocks, or cavities in pegmatites. It crystallizes from hot aqueous solutions. It forms at 90° to 250° C., under lower pressure and higher temperature than is normal at the depth where crystallization takes place. The color of pale amethyst is deepened by radium radiation, and decolorized specimens become violet again. However, naturally colorless quartz rarely becomes violet when radiated, but usually takes on a smoky brown coloration. Amethyst does not noticeably scatter transmitted light. The violet color is unstable at 260°, when amethyst becomes colorless. When hot, below 260°, it is gray violet, but resumes its original color on cooling. Manganese and titanium cannot be the coloring agents, since they are present only in small amounts which do not vary with the depth of color. It is concluded that amethyst is colored by a compound of ferric iron, stable up to 260°, and causing absorption of light at 0.53-.54μ. Other hypotheses of the cause of color in this mineral are not in accord with the experimental evidence. The chief points favoring a ferric pigment, briefly stated, are: a) Iron minerals are characteristically associated with amethyst. b) The absorption maximum of amethyst is very near that of some ferric compounds. c) Certain ferric compounds have the same color as amethyst. d) Upon being heated, darker amethysts become identical in color and absorption spectrum with citrine, which is thought to be colored by a ferric compound. e) The amount of iron in amethyst is large, and it varies in porportion to the depth of color. X. CHRONOLOGICAL BIBLIOGRAPHY OF IMPORTANT PAPERS ON SMOKY QUARTZ AND AMETHYST 1821. 1. BREWSTER, DAVID. On circular polarization, as exhibited in the optical structure of amethyst, with remarks on the distribution of coloring matter in that mineral. Trans. Roy. Soc. Edinburgh., 9,139-152. 1841. 2. POGGENDORFF, J. C. Formation of iron acids by a galvanic process. Pogg. Ann. Physik, 54, 353-377. 1845. 3. WOLFF, EMIL. The loss on ignition of quartz and fluorite. J. prakt. Chem., 34, 237. 1854. 4. HAIDINGER, W. The pleochroism and crystal structure of amethyst. Sitzb. k. Akad. Wiss. wien. Math.-naturew. Cl., 12, 401-421. 1855. 5. DESCLOIZEAUX, A. Memoir on the crystallization and internal structure of quartz. Ann. chim. phys., 45, 129316. 1860. 6. HEINTZ, W. The coloring constituents of flint, carnelian, and amethyst. Ann. Physik Chem., 60, 519-527. 1871. 7. FORSTER, A. The coloration of smoky quartz. Pogg. Ann. Physik, 143; 173-194. 1881. 8. HAWES, G. W. On liquid carbon dioxide in smoky quartz. Am. J. Sci., 21, 203-209. 1881. 9. WRIGHT, A. W. The gaseous substances contained in the smoky quartz from Branchville, Connecticut. Am. J. Sci., 21, 209-216. 1883. 10. BOKLEM, H. On amethyst. Neues Jahrb. Mineral. Geol., 1, 62-73. 1887. 11. ENGLER, C., and KNIES, E. Oxygen transfer and the solvent effect of liquid carbon dioxide on metals. Dingl. prakt. J., 263, 193-200. 1888. 12. JUDD, JOHN W. Development of a lamellar structure in quartz crystals by mechanical means. Mineralog. Mag., 8, 1-9. 1890. 13. DUFET, H. Comparative measurements on the indices of different quartz specimens. Bull. soc. franc. mineral., 13, 271-276. 1892. 14. JUDD, JOHN W. Additional notes on the lamellar structure of quartz crystals and the methods by which it is developed. Mineralog., 10, 123-135. 1896. 15. WEINSCHENK, E. The dilute colorings of minerals. Z. deutsch. geol. Ges., 48, 704-712. 1896. 16. WEINSCHENK, E. The mineral deposits of the Gross-Venediger stock in the Hohen Tauern. Z. Kryst., 26, 337508. (Smoky quartz, 394-403). 1896-7. 17. HLAWATSCH, C. The indices of refraction of some pigmented minerals. Z. Kryst., 27, 605-607. 1899. 18. KRAATZ-KOSCHLAU, K. v., and WÖHLER, L. The natural colorings of minerals. Tscher. Mineral. Petr. Mitt., 18, 304-333. 1899. 19. KRAATZ-KOSCHLAU, K.. v., and WÖHLER, L. The natural colorings of minerals. Tscher. Mineral. Petr. Mitt., 18, 447-468. JOURNAL MINERALOGICAL SOCIETI' OF AMERICA 251 1899. 20. NABL, ARNOLD. The coloring constituents of amethyst, citrine, and burnt amethyst. Sitzb. k. Akad. Wiss. Wien. Math.-naturw. Cl., 108, 2, Abt. b, 48-57. 1900. 21. KOENIGSBERGER, J. The coloring agent in smoky quartz. Tscher. Mineral. Petr. Mitt., 19, 148-154. 1900. 22. NABL, ARNOLD. Natural colorings of minerals. Tscher. Mineral. Petr. Mitt., 19, 273-276. 1900. 22. WEINSCIIENK, E. Natural colorings of minerals. Tscher. Mineral. Petr. Mitt., 19, 144-147. 1901. 24. KOENIGSBERGER, J. Mineral deposits in the biotite-protogene of the Aar massif. Neues Jahrb. Mineral. Geol., Beil.-Bd., 14, 43-119. 1901. 25. LACROIX, A. (Structure of amethyst). Mineralogie de la France, etc., 3, 41-45. Paris. 1904. 26. HINTZE, C. (Occurrences of smoky quartz and amethyst). HANDBUCH DER MINERALOGIE, 1, 1346-1441. . 1905. 27. EGOROFF, N. Dichroism produced by radium in colorless quartz, and a thermo-electric effect observed on the colored striae. Compt. Rend., 140 1027-1028. 1906. 28. BERTHELOT, M. Synthesis of amethyst quartz. Compt. Rend., 143 477-488. 1906. 29. MIFTHE, A. The coloring of precious stones by radium. Ann. Physik, (4), 19, 633-638. 1906. 30. WÜLFING, E. A. Mineral pigments. Festschr. z. 70 Geburtstag, II. Rosensbusch, 49-67. 1907. 31. BERRHELOT, DANIEL. The coloration of certain precious stones under the influence of radium. Compt. Rend., 145, 818-820. 1908. 32. DOELTER, C. The effect of radium and Roentgen rays on the colors of precious stores. Sitb. k. Akad. Wiss. Wien, Math.-naturw., Cl. 117, Abt. 1, 819-844. 1908. 33. DOELTER, C. The effect of radium and, ultraviolet light on mineral colors. Sitzb. k. Akad. Wiss. Wien, Math.natatrw. Cl., 117, Abt. 1, 1275-1325. 1908. 34. HERMANN, WALTER. The effect of oxidizing and reducing gases on the color of some minerals. Z. an.org. Chem., 60, 369-404. 1903. 35. PHILLIPS, C. E. S. Coloration of glass and quartz by radium. Nature, 77 535. 1908. 36. SIMON, KARL. Mineral colors. Neues Jahrb. Mineral. Geol., Beil.-Bd., 26, 249-295. 1909. 37. BRAUNS, R. The effect of radium rays on the color of sanidine, zircon, and quartz. Centr. Mineral. Geol., 721728. 1909. 38. DOELTER, C. The stability of mineral colorations produced by radium. Centr. Mineral. Geol., 232-234. 191.0. 39. DOELTER, C., and SIRK, H. The different effects of alpha, beta, and gamma rays on the colors of solids. Sitb. k. Akad. Wiss. Wien, Math.-naturw. Cl., 119, Abt. 2a, 1091-1100. 1910. 40. DOELTER, C. DAS RADIUM UND DIE FARBEN. 124-125. Dresden. 1913. 41. KOENIGSBERGE R, J. A classification of the east Alpine mineral deposits. Z. Kryst., 52, 151-174. 1915. 42. DOELTER, C. DIE FARBEN DIE MINERALIEN, 70-75. Brauntschuweig. 1917. 43. WATSON, T. L., and BEARD, R. E. The color of amethyst, rose, and blue varieties of quartz. Proc. U. S. Nat. Museum, 53, 553-563. 1918-19. 44. KOENIGSSERGER, J. Alpine mineral deposits. Abh. k. Bayer. Akad. Wiss., Math.-phys. Kl., 28, Abh. 10, 11, and 12 (parts I, II, and III). 1918. 45. NEWBERY, E., and LUPTON, H. Radioactivity and the coloration of minerals. Mem. Proc. Manchester Lit. Phil. Soc., 62, 1-16, Nature, 101, 198. 1919. 46. STRUTT, R. J. The scattering of light by solid substances. Proc. Roy. Soc. London, 95A, 476-479. 1920. 47. JOHNSEN, A. The paragenesis of alpha quartz and carbon dioxide. Sitzb. Bayer. Akad. Wiss., Math.-Phys. Kl., 321-328. 1921. 48. RAMAN, C. V. Smoky quartz, Nature, 108, 81. 1922 49. LACROIX, A. (Smoky quartz and amethyst in Madagascar). MINERALOGIE DE MADAGASCAR, 1, 196 et seq. Paris. 1922. 50. MEYER, S., and PRZIBRAM, K. Coloring and luminescence produced by Becquerel rays. Z. phys. Chem., 100, 334-336. 1922. 51. TUTTON, A. E. H. (Structure of amethyst), CRYSTALLOGRAPHY AND PRACTICAL CRYSTAL MEASUREMENTS, 1, 508-510. London. 1922. 52. WILD, G. O., and LIESEGANG, R. E. The colorings of some quartz varieties and their instability. Centr. Mineral. Geol., 481-483. 1923. 53. DOELTER, C. Color changes of minerals by radiations. Centr. Mineral. Geol., 321-324. 1923. 54. LIND, S. C., and BARDWELL, D. C. The coloring and thermophosphorescence produced in transparent minerals and gems by radium radiations. J. Franklin Inst., 196, 375-390; Am. Min., 8, 171-180. 1923. 55. VANZETTI, B. L. The colloidal nature of some coloring substances in minerals, and the possibility of determining their chemical nature by a spectrophotometric study. Atti. congresso naz. chim. pura applicala, 419, (1923). 1923. 56. WILD, G. O., and LIESEGANG, R. E. The color of amethyst and beryl. Centr. Mineral. Geol., 737-740. 1924. 57. TUTTON, A. E. H. (Structure of amethyst). THE NATURAL HISTORY OF CRYSTALS, 206-213. London. 1924. 58. VANZETTI, B. L. The color of smoky quartz. Gazz. chim. ital., 54, 95-99. While this dissertation was going through the press word was received of the tragic death of Dr. Holden who was drowned on August 5th at North Deer Isle, Maine.
*From a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the University of Michigan, June, 1925. That portion of the thesis dealing with the pigment of amethyst was submitted to the Boston Society of Natural History and was awarded the Walker prize for 1925. 1 The numbers in parentheses refer to entries in the bibliography, section X. 2 Robert Ridgway. COLOR STANDARDS AND COLOR NOMENCLATURE. Washington, 1912. 6 H. V. Ellsworth: Summ. Rept. Geol. Surv. Canada 1921, pt. D, 51-70. 7 A. Lacroix: MINERALOGIE DE MADAGASCAR, 11, 260 (1922). 10 F. E. Wright and E. S. Larsen: Am. J. Sci., 27, 421-447 (1909). 11 H. C. Sorby. Quart. J. Geol. Soc.; 14, 453-500 (1858). 12 E. H. Amagat: Compt. Rend., 114, 1093-1098 (1892). 14 Waldemar Lindgren: MINERAL DEPOSITS, 2nd edition, 468 (1919). 15 C. DOELTER: HANDBUCH DER MINERALCHEMIE, 11, pt. 2, 555 (1917). 16 R. A. Daly: Proc. Nat. A cad. Sci., 3, 659-665 (1917). 20 Well shown for FeCl3 in plate 51A of H. C. Jones and J. A. Anderson's Absorption Spectra of Solutions: Carnegie Inst. Publ., 110, 1909. 21 Jane Bonnell and Edgar Perman: J. Chem. Soc., 119, 1994 (1921). 23 Edw. F. Holden: Am. Min. 9, 75-88, 101-108 (1924). 24 U. S. Bureau of Mines Bull. 212, 230 (1923). 25 R. J. Strutt: Proc. Roy. Soc., 80A, 588 (1908). 26 Edw. F. Holden: Am. Min. 9, 75-88, 101-108 (1924). 28 Cf. S. C. Lind: J. Phys. Chem., 24, 437-443 (1920). 29 Edw. F. Holden: Am. Min. 9, 75-88, 101-108 (1924).
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||