| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
Volume 17, pages 541-550, 1932 A NEW OCCURRENCE AND X-RAY STUDY OF MOSESITEPAUL H. BIRD, Columbia University. INTRODUCTION Mosesite from Terlingua, Texas was originally described by Canfield, Hillebrand and Schaller.1 The mineral was found in association with eglestonite, terlinguaite, montroydite and kleinite in the quicksilver mining district. Soon after the original description appeared, Schaller2 made a more thorough and extended study of the mineral. Since that time, however, search of the literature indicates the absence of any record of the occurrence of mosesite at any other locality. Recent studies, however, have demonstrated the presence of the mineral in association with the mercury minerals of the T. S. Clack Quicksilver Mine in the Fitting District, Nevada. It appears that a description of the new occurrence of this rare mineral is worthy of record. In addition to the description of a new locality for mosesite the study has provided x-ray data which may be used in distinguishing mosesite from the minerals with which it has been found to occur. Dr. Paul F. Kerr, of Columbia University, in company with Mr. C. D. Woodhouse, general manager of Champion Sillimanite Co., collected the specimens upon which this study is based during the summer of 1931. Dr. Kerr has also offered suggestions and has assisted in taking x-ray photographs. Dr. A. F. Rogers, of Stanford University, has kindly read the manuscript and offered valuable suggestions. Mr. A. M. Smoot, of Ledoux and Co., has generously assisted the investigation through his painstaking procedure and personal attention he has given to the chemical analysis. LOCATION AND OCCURRENCE The Clack Quicksilver Mine where the specimens of mosesite were found is situated on the eastern side of the mountainous terrane between Star Range and Buffalo Mountain in Pershing County, Nevada, about twenty-three miles northeast of the town of Lovelock. The elevation above sea level is approximately 5500 feet. The discovery upon which the present quicksilver mining operations are based was made in 1927. A small amount of high grade ore, however, had been previously taken from a prospect shaft. The chief ore mineral is cinnabar, and the average ore carries between 2 per cent and 3 per cent of mercury. The mine is located on a hillside, and may be entered either from an untimbered shaft extending downward 350 feet from the surface, or from the haulage tunnel on the 350-foot level. It is estimated that between 1000 and 1500 flasks of mercury have been extracted since the mine was opened. The country rock is a fine-grained, massive, medium-gray limestone, probably Tertiary in age. No evidence of fossiliferous remains were observed in the specimens studied, but it has been reported that a few fossils have been found in the vicinity. A microscopic study of thin sections shows that the rock has been subjected to intense deformation. Places may be observed where evidence of four distinct periods of fracturing appear within one field of view. The fracturing has been so severe and thorough that it is difficult to find a field of view that does not show several fractures. The rock is essentially a pure limestone with a very minor amount of quartz. The latter occurs in two distinct forms. An occasional grain may be observed that is well rounded, and has apparently had no connection with the fracturing and mineralization of the rock. These are interpreted as original fragmental grains deposited with the limestone. In addition, patches and streaks of very small, euhedral quartz crystals are sparsely distributed throughout the rock. An interesting but minor feature is the occurrence in these crystals of minute, detached particles of calcite distributed in planes parallel to the crystal faces. It appears that the quartz forming these crystals was introduced by solutions contemporaneously with the fracturing and healing of the rock. The cinnabar together with crystalline calcite occurs as a replacement in veins and in small irregular masses in the limestone. The gangue is probably more than 95 per cent calcite, the latter occurring as a microscopically coarse crystalline mass. The only other gangue mineral is quartz. In occasional instances areas may be observed in which the quartz exhibits the comb structure of vein quartz. Several small areas were noted which suggest that part of the quartz is genetically related to the cinnabar. Native mercury occurs in small openings in the limestone. Specimens of the unusual mercury mineral mosesite occur along with native mercury. The latter, however, is found only in a few places, near the surface and in small concentrations. MOSESITE OCCURRENCE. In the original description of mosesite from Terlingua, Canfield3 comments upon the mineral as occurring in small yellow crystals perched upon crystals of calcite. He states that the crystals occur isolated and solitary, and appear to lie upon the calcite with little or no bond as if the slightest touch would loosen them, leaving no scar upon the calcite but merely a clean spot. The description of the occurrence of mosesite at Terlingua agrees in general with the description of the occurrence of this mineral as it is found in the Fitting District. In Nevada, however, the crystals occur chiefly in small groups of two or three, the individuals of which are intergrown in a very irregular manner. GENERAL DESCRIPTION. No attempt has been made to measure the interfacial angles by means of the goniometer on account of the small size of the crystals available, and their apparent irregularities. Schaller4 experienced considerable difficulty in obtaining accurate measurements of the crystals from Terlingua, and similar difficulties seem inescapable in the case of the specimens from the Fitting District. Study under a binocular microscope and with a hand lens indicates that the crystals from the latter place are chiefly octahedra, in some cases slightly modified by small dodecahedral faces. A few crystals with a predominant cubic habit have been observed. Schaller5 noted the probable occurrence of twinning according to the spinel law in the Terlingua material, and the same appears to apply to that from the Fitting District. The color of the crystals studied is a pale canary yellow when taken from the mine. This color will change to a light olive green after two months' exposure in ordinary room light. This observation differs from that of Canfield,6 who stated in his original description of the mineral that there were no indications that the crystals were affected by light. He did admit, however, that "If the light affects the color, it must act very slowly." One of the best specimens in the mineral suite from the Fitting District was left exposed to ordinary room light, but protected from the direct rays of the sun, and observed every few days and compared with a specimen that was kept in the dark. The change in color was not noticeable for at least a month, and even then progressed very slowly. Mosesite from either Texas or Nevada does not appear to change color on grinding. The closely related mineral eglestonite from Terlingua, Texas, turns dark gray when finely powdered.7 Several of the crystals were crushed and mounted in Canada balsam for microscopic study. Apparently the optical properties agree with those given by Schaller. The crystals are pale yellow in color, and show no pleochroism. They exhibit at least a strong suggestion of octahedral cleavage, and possess a rather uneven birefringence in polarized light. Schaller found that when heated above 186 degrees centigrade the mineral becomes isotropic, and therefore corresponds optically with the observed crystal form only at this higher temperature. The observed hardness, luster, streak, and brittleness appear to agree with the recorded observations of Canfield, Hillebrand, and Schaller.8 CHEMICAL ANALYSIS. A small sample, weighing only 5.88 milligrams, was submitted to Mr. A. M. Smoot of Ledoux and Company, New York City. Mr. Smoot very kindly performed all the work personally, realizing the difficulties that were to be expected in working with such a small amount of material. His results are given in Table 1. In commenting on the accuracy of this analysis, he says: "The mercury determination may be three or four per cent out of the way; the chlorine may be one per cent out, one way or the other. The SO 4 determination and the NH3 determination I believe are fairly close, considering the small amount of sample available." The chief discrepancy between these results and those of Hillebrand,9 it will be noted, is in the SO4 content. Hillebrand's analysis showed 32 per cent of SO4; just half that shown in Mr. Smoot's analysis. However, Hillebrand states that his values are no more than approximations, which coupled with the difficulties encountered by Mr. Smoot, indicates that too much stress should not be placed on the quantitative results obtained. The real significance attached to the results of Mr. Smoot lies in the fact that he established the presence of mercurous mercury in the mineral, presumably with mercury bound to chlorine. In this connection Hillebrand states: "It will be remembered that the chemical evidence points to kleinite being a mixture of mercury-ammonium chloride with a mercuric sulphate and perhaps chloride. There would seem to be an association of a similar character in mosesite, but with a mercurous sulphate or chloride replacing in part or wholly the corresponding mercuric salts of kleinite." This difference he accepts as a certain means of distinguishing mosesite from kleinite. Since the mercurous form of mercury was definitely proved to be present, and since the other elements correspond qualitatively, and as closely as could be expected quantitatively with those found in mosesite as originally described, it is concluded on chemical grounds that the mineral from the Fitting District is mosesite.TABLE 1 CHEMICAL ANALYSIS OF MOSESITE FROM THE FITTING DISTRICT, NEVADA*
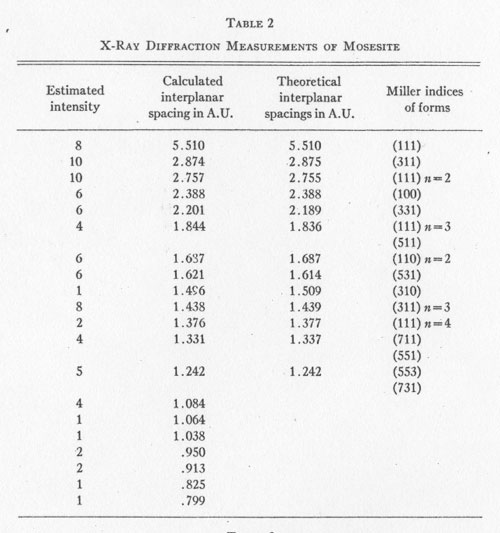
Mercury (Hg).................... 83.00%+ * By Mr. A. M. Smoot of Ledoux and Co., New York City X-RAY ANALYSIS. A preliminary examination of the material from Nevada led to the opinion that the small yellow crystals might be eglestonite. Accordingly several x-ray diffraction patterns of the material were compared with x-ray diffraction patterns of eglestonite from the Egleston Collection of Columbia University. This comparison showed conclusively that the mineral from Nevada was not eglestonite. This preliminary investigation indicated that 15 milligram samples were close to the optimum amount for the production of clear, distinct patterns. Specimens of kleinite, terlinguaite, montroydite, and mosesite from the Texas locality were then prepared for x-ray examination, and diffraction patterns made of each. The results were very gratifying, and furnished what is considered the most reliable data of any of the methods of determination attempted. The pattern of mosesite from Terlingua and that of the mineral from the Fitting District were identical with the exception of the intensity of one line, which was a trifle fainter in the one from the latter locality. Figure 1 is a reproduction of the two patterns side by side. The Miller indices of the atomic planes represented in the patterns of eglestonite and mosesite were determined by means of the Cubic Crystal Analyser.10 Mosesite appears by this means of analysis to have a unit cell with a diamond type of space lattice, while eglestonite has a unit cell with atoms arranged according to the simple cube space lattice. The unit cube of mosesite measures approximately 9.55 Angstrom units on a side, and that of eglestonite approximately 9.50 Angstrom units. The computations of the interplanar spacings were checked with the values determined by calculation based on the theoretical ratios of the various interplanar spacings to each other. It was found in determining the Miller indices of the atomic planes represented that there was one line in the eglestonite and one in the mosesite pattern that did not correspond to any possible plane that could exist in harmony with the isometric symmetry. It was this particular line that appeared a little more intense in the pattern of mosesite from Terlingua than in that from the Fitting District, as already stated. Upon further investigation it was found that a line appeared in about the same position in the patterns of all the other minerals of this group, but with much greater intensity in the pattern of montroydite, in which it was the most intense line. This fact would appear to indicate the presence of a trace of montroydite in the other minerals of the group. This line is not shown in the tables or chart for eglestonite and mosesite. TABLE
2 TABLE
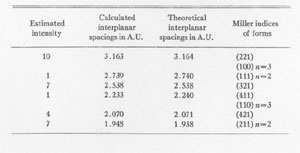
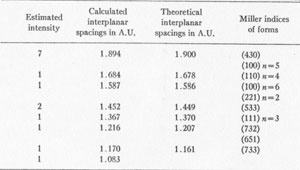
3
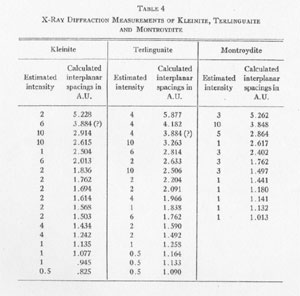
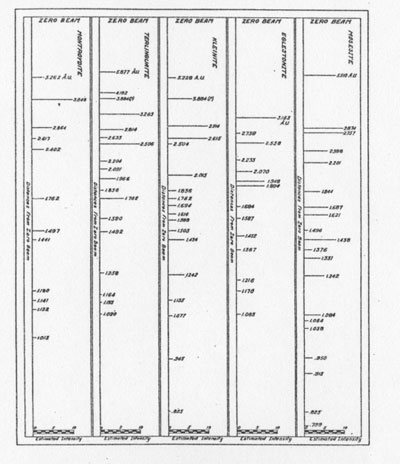
The results of the x-ray work on all five of these rare minerals are given in Tables 2, 3, and 4. Table 2 gives the estimated intensity, calculated and theoretical interplanar spacings, and Miller indices of the planes represented for mosesite. Table 3 shows the same for eglestonite. Table 4 shows the estimated intensity and interplanar spacings for terlinguaite, montroydite, and kleinite. The calculation of each interplanar spacing for all the minerals was checked against sodium chloride and corrected. Figure 2 is a chart presenting the x-ray data in graphical form. The measurement of the arc for each line as taken directly from the diffraction pattern is plotted along the horizontal lines, and a vertical line drawn at each point so determined. The length of each vertical line represents the estimated intensity of the line which it represents in the diffraction pattern as shown by the scale. At the end of each vertical line is given the interplanar spacing calculated for that line and corrected with the known theoretical value of a line occupying approximately the same position in a pattern of sodium chloride taken on the same strip of film. CONCLUSION Studies based chiefly on data obtained by x-ray and chemical analyses indicate the occurrence of mosesite in the Clack Quicksilver Mine, in the Fitting District, Nevada. In connection with this investigation x-ray analyses were also made of the other rare mercury minerals, namely: eglestonite, terlinguaite, montroydite and kleinite, which were first described from Terlingua, Texas. Each of these minerals gave a characteristic and distinctive diffraction pattern, the measurements of which are recorded in the preceding pages.
NOTES 1 Canfield F. A., Hillebrand, W. F., and Schaller, W. T., Mosesite, a New Mercury Mineral from Terlingua, Texas: Am. Jour. Sc., 4th. Ser., vol. 30, pp. 202-208, 1910. 2 Schaller, W. T., Bull. 509 U.S.G.S., pp. 104-109, 1912. 3 Canfield, Hillebrand, and Schaller, op. cit. 4 Schaller, W. T., op. cit. 5 Schaller, W. T., op. cit. 6 Canfield, Hillebrand, and Schaller, op. cit. 7 Eglestonite from Redwood City, California, retains the light color when finely powdered. 8 Canfield, Hillebrand, and Schaller, op. cit. 9 Canfield, Hillebrand, and Schaller, op. cit. 10 Made by Adam Hilger, London, England. |