| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
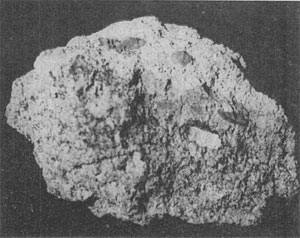
Volume 48, pages 620-634, 1963 NATURAL α-SILICON CARBIDE J. BAUER, J. FIALA AND R. HRICHOVÁ, Department of Mineralogy, Institute of Chemical Technology, Prague, Czechoslovakia. ABSTRACT A second natural occurrence of α-silicon carbide discovered in Bohemia is in nonkimberlitic volcanic breccia in a region of kimberlitic rocks. The only other known natural occurrence of α-SiC is in a kimberlite diamond pipe in Siberia. All crystals conform to the structural hexagonal type II(6H), except one crystal which is a combination of types I(15R) and II(6H). The refractive indices are ω=2.647 and ε=2.689, and the specific gravity is 3.23-3.24. Lattice dimensions are: a=3.06 Å and c= 15.18 Å. The character of luminescence does not correlate with the structural type as previously reported. The morphological, physical and chemical properties of natural moissanite and synthetic silicon carbide agree very closely. INTRODUCTION The natural occurrence of silicon carbide was first reported by Moissan (1905) from the Canyon Diablo meteorite. It was first found in terrestrial rocks in the Green River Formation of Wyoming by Regis and Sand (1958) and was identified as the low temperature cubic ß-SiC polymorph. Another polymorph, hexagonal α-SiC, was described by Bobrievitsch et al. (1959) from the diamond pipes of Yakutia. The second occurrence of natural α-SiC reported in this paper was found in 1960 in a newly discovered volcanic breccia in the northwestern part of Bohemia in a region of kimberlite rocks. VOLCANIC BRECCIA. The light colored breccia, tinged brownish-yellow, is composed mostly of fragments of volcanic material and weathered chalk-marl (Fig. 1) the latter penetrated by limonite plates. Other rock types in the breccia include carbonaceous sandstone, faintly granular fossiliferous limestone, garnet gneiss, Tertiary basalt and large limonite concretions. The heavy mineral concentrate from the breccia consists of limonite, pyrope-almandite garnet with the almandite molecule dominant, kyanite, pleonaste, magnetite, diopside, pyroxene, apatite, rutile, sphene, olivine, zircon, pyrite, galena, fluorite, metallic iron and moissanite. Light minerals are quartz, feldspar, white mica, calcite and gypsum. This breccia cannot. be classified as a kimberlite breccia, because of the lack of such characteristic minerals as pyrope, ilmenite, the chromspinelides, phlogopite and chromdiopside. According to Kopecky (1960) the breccia is related genetically to the Late Tertiary alkali vulcanism of the Bohemian Mittelgebirge. It has a texture very similar to that of the kimberlite breccias of the region and is surrounded by chalk-marl except at its western margin where Tertiary diatomite is present.
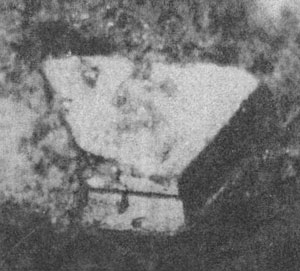
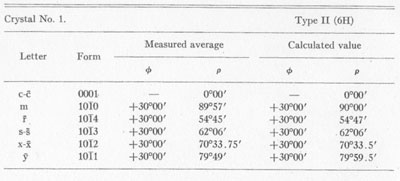
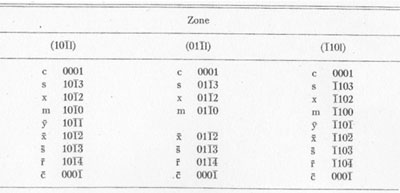
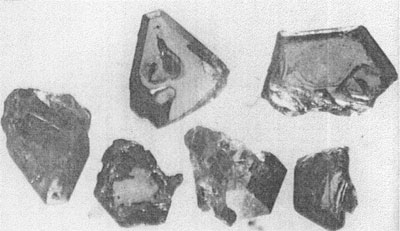
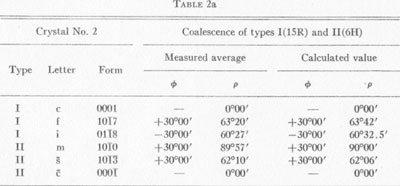
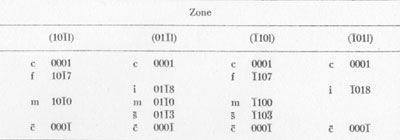
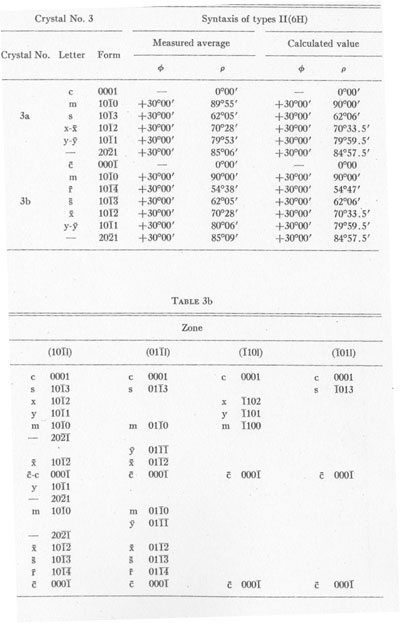
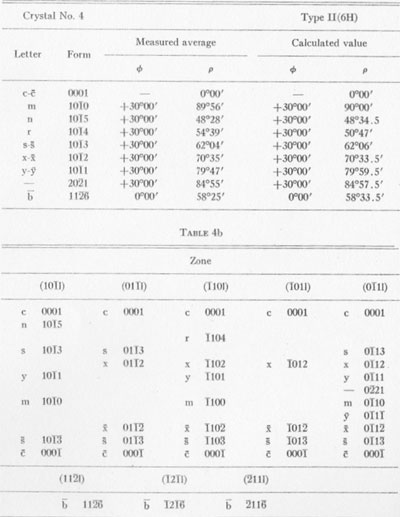
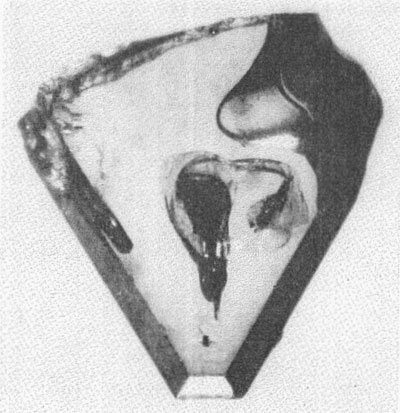
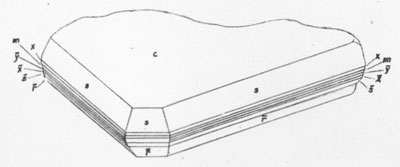
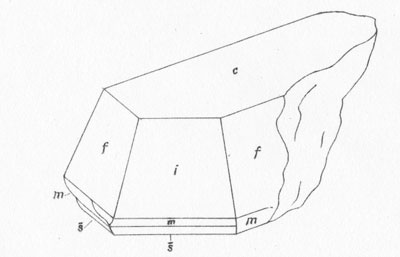
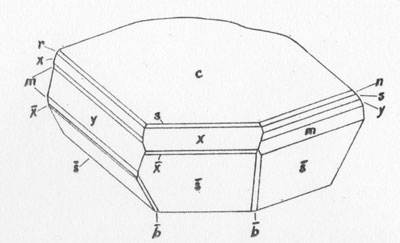
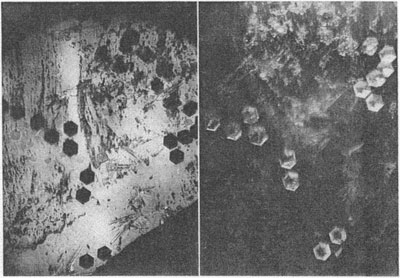
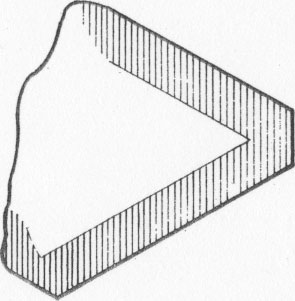
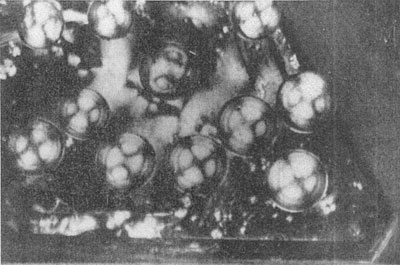
MOISSANITE Crystallography. Some grains of moissanite are associated with thin laminae of limonitic material penetrated by metallic iron (Fig. 2). Others are more or less idiomorphic (Fig. 3), and still others are irregularly rounded, small fragments of larger crystals. The grain size ranges mostly from 0.3 to 1.8 mm; 1 mm-size grains dominate. Idiomorphic crystals are tabular basal pinacoid forms (Fig. 4). Relatively common are grains which are half bounded by crystal faces and half irregularly rounded. On the pyramid faces of several crystals, conical protuberances are bounded by an irregular face. Some crystals show parallel growth emphasized by a seam (Fig. 2). In all cases the basal face appears as a composite plane. Many of the moissanite grains are characterized by perfectly smooth, lustrous surfaces. A few thick tabular crystals have matte surfaces, on pyramid faces only. Several crystals were measured on the two-circle goniometer. All conform to the structural hexagonal type II(6H), except crystal No. 2 which exhibits a combination of types I(15R) and II(6H). Crystal No. 3 is represented by a syntaxis of type II(6H). Morphological data are given in Tables la to 4a and the distribution of forms in different zones is given in Tables 1b to 4b. The axial ratio a:c=1:4.9070 and po:ro=5.6661:1 was taken for type II; a:c=1:12.2675, po:ro=14.1653:1 for type I. Perspective drawings of measured crystals (Figs. 5 to 7) bear letter designations for faces based on the detailed study of Thibault (1944). All the forms described by Thibault were ob served on natural moissanite. In two cases the form 2021 was observed which Thibault considers as an uncertain form for synthetic α-SiC. A second-order pyramid b (1126) was observed only once. Only f (1017) and i (0118) were observed on type I of crystal No. 2, which is a combination of types I and II. The present study of natural moissanite as well as that of synthetic α-SiC indicates that morphological criteria for the distinction between upper and lower forms are inadequate, and that etching is necessary for their reliable distinction. Crystals of moissanite were etched using a melt of NaNO3+Na2CO3; are shown in Fig. 8.
TABLE la
TABLE 1b
TABLE 2a
Specific gravity. The specific gravity has been determined by suspension of the moissanite in Clerici solution. Fragments of all shades of color were examined. σ/20° C.=3.23-3.24. Optical properties. Numerous crystals have been examined for color, luster, pleochroism, transparency, luminescence, optical character and optical anomalies. These properties for the four most interesting crystals are described in Table 5. The dichroscopic ocular was used to observe pleochroism. As a rule, pleochroism of intensely colored grains is very weak. Some crystals composed of two variously colored parts have distinctly different pleochroism. Luminescence was investigated using ultra-violet light (Philora λ =3.650 Å). It was found that the intensity of yellow luminescence is greater the fainter the color of the grain. The luminescence of dark colored specimens is very weak, orange-yellow, or not apparent. Some light bluish-green crystals display a sharply bounded intense yellow stripe at their margins, as shown in Fig. 9. Inclusions have no apparent effect on the character of luminescence.
TABLE 2b
TABLE 3a
Dietzel, et al. (1959), who made a detailed study of luminescence of synthetic α-SiC, also observed reddish-violet and blue luminescence in several cases. According to them, yellow luminescence is associated with the structural type I (15R), and blue with type II(6H). These authors also determined that increase of intensity of luminescence correlates with decrease in grain size.
TABLE 4a Optical anomalies are obvious with both orthoscopic and conoscopic observation. Anomalous extinction in the direction of the c-axis was observed on some crystals. By use of the microconoscopic method (Bauer, 1958) it is possible to investigate anomalous biaxiality which is especially apparent around inclusions (Fig. 10).
The refractive indices measured on tabular grains by means of de Chaulnes' method as well as by the prismatic method give the following values for Na light: ω=2.647 ε=2.689 The optical character in all cases is positive.
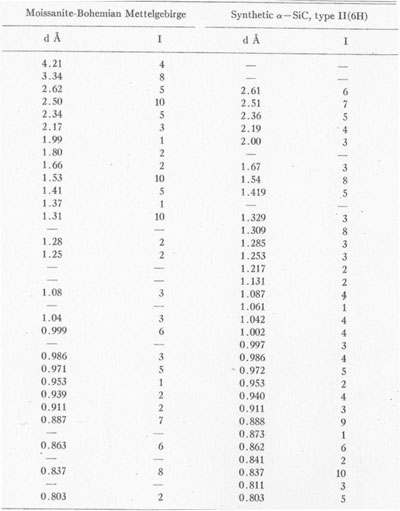
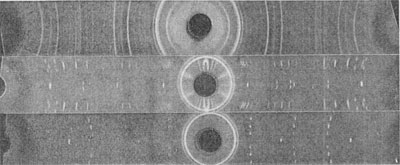
X-ray data. For preliminary identification a powder photograph was obtained using filtered CuKα radiation (Fig. 11). Data from the powder photograph are compared with those obtained by Thibault (1944) for synthetic Table 6 shows the close comparison between natural and synthetic α-SiC, type II(6H).
For precise determination of the lattice dimensions (a, c), rotation photographs were made with rotation about axes a and c (Fig. 12, 13) and the following values were found: a=3.06Å c=15.88Å
a=3.073Å c=15.08Å Borrmann and Seyfarth (1933) give the following values obtained from a powder photograph: a1 =
a2 = 3.076 ± 0.003Å
TABLE 5
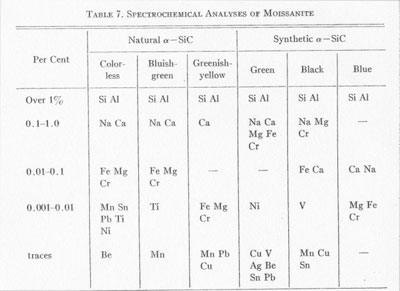
Ott (1925) by precise measurement found values for this same type of photograph of: a=3.095Å c=15.17Å Spectrochemical analyses. Spectrochemical analyses (Table 7) were made for both natural and synthetic α-SiC using a continuous arc between carbon electrodes, in a Q-24 spectrograph with quartz optics. Lundgvist (1948) stated that there is a close correlation between the aluminum content and the structural type, for example concentrations of less than 0.01% Al result in type II; higher concentrations of Al result in type III.
TABLE 6
The spectrochemical analyses (Table 7) show that the content of Al for all colors is higher than 1% in our samples and that there is no relationship between structural type and color. Also, Ott (1926) did not mention such a correlation, but did state that the relationship between trace element content and structural type is very problematic.
TABLE 7 . SPECTROCHEMICAL ANALYSES OF MOISSANITE
After systematic comparisons between color of silicon carbide and Fe, Ti and V impurity content, Dietzel et al. (1959) found no relationship. They also pointed out that the light-colored greenish-yellow silicon carbide was obviously formed in the highest temperature part of the furnace. Homogeneity of crystals. Clear, homogeneous crystals of moissanite are very rare. Inclusions are numerous in most cases. These are in part black, opaque, and of irregular shape as seen centrally located in Fig. 4 or are black, opaque and enclosed in crystallographically bounded cavities. In the synthetic product such inclusions are considered by many authors to be graphite. Inclusions of another type, represented by needle-shaped idiomorphic crystals, are transparent and brown or orange-brown in transmitted light. A special type of very rare inclusion is represented by spheres of metallic iron partially embedded in the moissanite. Such inclusions have never been described with synthetic SiC. Lundqvist (1948) mentioned only the separation of iron carbides and silicides within or on the surface of crystals with increasing Fe content in the melt. DISCUSSIONS AND CONCLUSIONS In Siberia moissanite has been isolated directly from kimberlite diamond pipes. In view of this occurrence and the presence in Bohemia of moissanite in non-kimberlitic volcanic breccia, it is very surprising that moissanite has not been reported from the South African kimberlites. The Bohemian moissanite is represented by a high-temperature α-SiC polymorph formed in the range from 1900 to 2000° C. This high temperature of formation and the presence of fragmental crystals indicate that the moissanite and associated volcanic breccia came from a great depth. However, the moissanite in Bohemia is associated with distinctly hydrothermal minerals such as galena, pyrite and fluorite which normally form at shallower depths. Regis and Sand (1958) believe the low-temperature ß-SiC polymorph to be of hydrothermal origin, even though its temperature of formation is between 1200 and 1300° C. Our findings for luminescence are not in accord with those published by Dietzel et al. (1959) who observed reddish-violet and blue luminescence in ultraviolet light. However, they did not report the wave length of the ultraviolet source. The character of luminescence does not correlate with the structural type, as is shown by the irregular distribution of luminescence in many crystals of the same structural type. The occurrence of two structural types of different color in the same crystal gives some indication of the very complex conditions under which the natural crystals formed. These conditions are not yet clearly explained even for synthetic material. Moissanite and synthetic SiC show no morphological differences even though natural moissanite crystals are less than 2 mm in size, whereas the synthetic product reaches several centimeters in size. The morphological, physical and chemical properties of moissanite and synthetic silicon carbide are in surprisingly good agreement. REFERENCES BAUER, J. (1958) Silikáty, 2, 178. BOBRIEVITSCII, A. L., M. N. BONDARENKO, M. A. GNEVUSCHEV, L. M. KRASOV, G. I. SMIRNOV, AND R. K. JURKEVITSCH (1959) Alnmznyye Meslorozdenija Jakuti, Moskva, 320. BORRMANN, G. AND H. SEYFARTII (1933) Zeit. Krist. 86, 472. DIETZEI., A., H. JAGODZINSKI AND H. SCHOLZE (1959) Untersuchungen an technischem Siliziumcarbid, Köln und Opladen. Forschungsberichte des Wirtschafts und Verkehrsministeriums Nordheini-Westfalen. KOPECKÝ, L. (1960) Izvestija ANSSSR. 12, 52. LUNDQUIST, D. (1948) Acta chem. Scandinavica, 2, 177. DIOISSAN, H. (1905) Comptes rendus, 140, 405. OTT, H. (1925) Zeit. Krist. 61, 515. --- (1926) Zeit. Krist. 63, 1. REGIS, A. J. AND L. B. SAND (1958) Bull. Geol. Soc. Am. 69, 1633. THIBAULT, N. NV. (1944) Am. Mineral. 29, 249. Manuscript received, October 1, 1962; accepted for publication, January 11, 1963.
| |||||||||||||||||||||||||||||||||||||