| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
Volume 56, pages 1319-1331, 1971
PAUL B. MOORE, The Department of the Geophysical Sciences, University of Chicago, Chicago, Illinois 60637 Abstract Copper nickel arsenides collected from a large fissure at the Mohawk No. 2 mine, Mohawk, Keweenaw Co., Michigan were investigated by powder diffractometry and electron probe analysis. These materials correspond to the "keweenawite" and "mohawkite" of Koenig and the "ledouxite" of Richards. The phases identified include α-domeykite, β-domeykite, algodonite, arsenical copper, rammelsbergite, pararammelsbergite and niccolite. Sealed silica tube studies in the system Cu-Ni-As at 510±5°C show negligible solubility of copper in the nickel arsenides and nickel in the copper arsenides. All examples encountered in the Mohawk disequilibrium assemblage appear to involve essentially no (Cu,Ni) solid solubility among the binary arsenide compounds. There is strong evidence that the nickel arsenides crystallized earlier al higher temperature, were fractured and subsequently partially replaced by the copper arsenide-bearing solutions at lower temperature. Reaction rims around the nickel arsenides represent products which are consistent with the inferred stability of coexisting phases in the Cu-Ni-As system. INTRODUCTION This paper describes and discusses the paragenesis of the coexisting copper-nickel arsenides which comprise the socalled "keweenawite" and "mohawkite" of the Mohawk No. 2 mine, Mohawk, Keweenaw Co., Michigan. Prior to the advent of the techniques of metallography and X-ray diffractometry, a spate of names appeared in the literature including "ledouxite" (Richards, 1901), "keweenawite", "mohawkite" and "whitneyite" (Koenig, 1900). All were eventually stated to be mixtures of at least seven known and unknown phases by Butler and Burbank (1929). Although these authors primarily discussed the copper arsenides, they mentioned the existence of several unknown nickel and cobalt arsenides. The now-inaccessible Mohawk No. 2 shaft was driven into a member of the Kearsarge Lode series of amygdaloidal basalt, and encountered unusually large quantities of copper arsenides, witnessed by the removal of over 230,000 pounds of "mohawkite" material (Butler and Burbank, 1929). A more recent list of copper-nickel arsenides from this general region was published by Williams (1963). Occurrences of coexisting copper-nickel arsenides are few and include Cerý, Dul, Krkonose, Bohemia where arsenic, arsenolamprite, niccolite, koutekite, paxite, and novakite were described by Johan (1962) and the Talmessi mine, Anorak, Iran from which niccolite, algodonite, and α-domeykite were identified by Bariand and Herpin (1960). To assist in the paragenetic interpretation of the Mohawk mine arsenides, the system Cu-Ni-As was investigated at 510±5°C by synthesis in evacuated sealed silica tubes. ARSENIDES OF THE MOHAWK NO. 2 MINE Phases encountered and described include α-domeykite (Cu3As), β-domeykite (Cu3-xAs), algodonite (Cu6±xAs), arsenical copper (Cu,As)s.s., rammelsbergite (NiAs2), pararammelsbergite (NiAs2), and niccolite (Ni1±xAs). Specimens. A representative suite of specimens was gathered in 1962 by the author from the 11th and 12th levels, 200 ft S.W. of the Mohawk No. 2 shaft. Since then, access to the shaft has been sealed and the neighboring mines with access routes have been abandoned. The arsenide-bearing fissure pinches and swells, in some places attaining a thickness of four feet, and is nearly completely filled with arsenides and considerable amounts of nearly pure calcite. Recent action by mine waters has produced numerous hydrated calcium and copper arsenates which frequently coat the calcite and the arsenides. In some places, calcite is the sole mineral. A history of brecciation is evident with angular pieces of wall-rock immersed within the calcite. A large stope, between the levels exposes the fissure, which shows veins of α-domeykite up to two feet in thickness. The material in place is of a pale chalcopyrite-yellow color, much like pyrrhotite in appearance; when freshly broken, samples afford the smell of arsine gas. When brought to the surface, α-domeykite characteristically tarnishes to a chalcopyrite-yellow color and develops within a few years a fuzzy black pulverulent coating not evident in situ which consists of at least two phases. One of these phases is pale green in color and yields a complex powder pattern similar to tyrolite, indicative of a product of oxidation. This coating can be observed on nearly all α-domeykites preserved in collections. It is likely that the presence of reducing mine gases such as methane inhibit the surficial oxidation of α-domeykite in place. Knots of greyish tin-white rammelsbergite of botryoidal and fibrous appearance occur in places, usually along the edge of the fissure, and these invariably show treelike fractures filled with α-domeykite. Polished sections of the "keweenawite" display rammelsbergite with a reniform or botryoidal outline, mimicking the appearance of the cobalt-nickel arsenides from the Cobalt-Sudbury region in Ontario. Masses up to six inches across consisting principally of nickel arsenides are not rare, but α-domeykite is by far the most abundant arsenide in the fissure. Pinkish niccolite usually separates the rammelsbergite from α-domeykite and occurs as thin zones to fairly large masses intimately mixed with rammelsbergite and α-domeykite. Often, the centers of the fissure fillings contain α-domeykite which passes laterally into α-domeykite-niccolite mixtures which in turn grade into niccolite-rammelsbergite mixtures. Figure 1 represents a typical appearance and shows the tree like α-domeykite bordered by niccolite which adjoins the rammelsbergite. These phases can be visually distinguished if the polished sections are allowed to tarnish in air. Rammelsburgite retains its tin-white appearance, niccolite appears brassy-pink, and α-domeykite assumes an oily-green tarnish. Sometimes these mixtures are so intimately mixed that resolution of the phases is prohibitive - even by probe techniques - and recourse to powder diffractometry is necessary. Although the neighboring large masses of α-domeykite appear to be constituted of pure material, rare tiny remnant grains of niccolite, arsenical copper, and algodonite were verified by probe analysis.
Fig 1. Photograph of α-domeykite-rammelsburgite-niccolite mixture which was polished and allowed to tarnish in air. The α-domeykite appears as the dark tree like outline, and the niccolite- rammelsbergite mixture is mottled. Occasional islands of pure rammelsbergite are white. The calcite which surrounds the sample and which occurs as rare late-stage veins is white. The sample is about 5 cm. across.
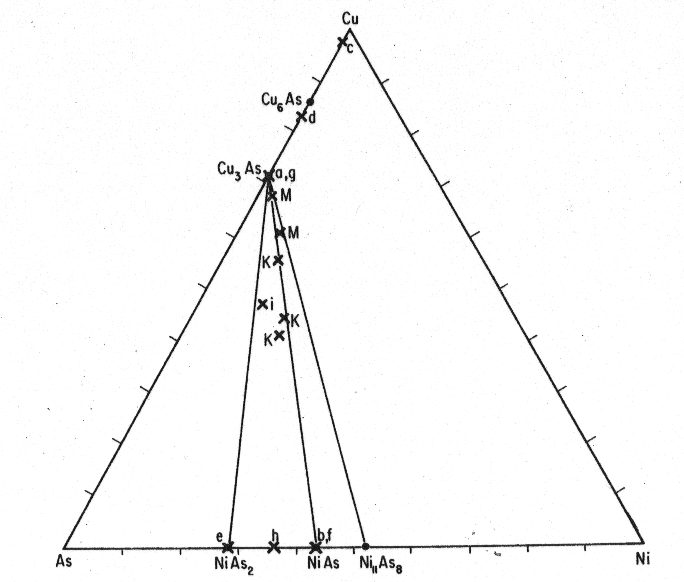
These rammelsbergite-niccolite-α-domeykite mixtures reveal a striking resemblance to Koenig's type specimens of "keweenawite" and "mohawkite" which he apparently collected from the same fissure intersecting the 5th level, and the intimate aspect of some of the mixtures doubtlessly led him to conclude that the materials were homogeneous. Koenig's location was inaccessible during the time of specimen collection due to extensive mine collapse. Less frequent species identified to the course of this study include pararammelsbergite , β-domeykite, algodonite, and arsenical copper. The algodonite and arsenical copper, rare at this locality, are commoner at some other mines. Enormous masses were once encountered at the Ahmeek No. 3 and 4 mines about a mile to the south and now lay buried in the waste heaps. The β-domeykite is usually intermixed with α-domeykite; however, a sample collected and kindly donated by G. Kullerud from the adjacent Seneca shaft consists essentially of β-domeykite. Maucherite was not encountered, but it occurs in some fissures at other locations in the Keweenaw Peninsula, usually with algodonite (R. J. Skinner, pers comm.). It may be remarked that the relative abundances of the Cu-Ni-As phases encountered even in one fissure may vary extensively and the assemblage reported herein is representative on only one specific location. However, it is believed that the detailed microparagenesis of the associated phases is essentially repeated at the other arsenide occurrences in the Keweenaw Peninsula since specimens of copper nickel arsenides from several other locations show the same associations when examined on a small scale. Experimental. ARL electron probe analysis and X-ray powder diffraction studies were undertaken on the natural samples. Studies in the Ni-As system, by Yund (1961) and the Cu-As system by Skinner, Adler, and Mead (1963) and Skinner and Luce (1971), and an isothermal section of Cu-Ni-AS system at 510°C, discussed subsequently, were invaluable aids in interpretation of the mineralogical relations. Koenig's bulk analysis of "keweenawite" and "mohawkite" fall within the Cu3As-NiAs2-Ni11As8 field as depicted in Figure 2 and samples investigated in this paper, with rare exception, also fall within this field. Two kinds of analyses were performed on the electron probe. Matrix absorption and enhancement calculations showed that no corrections were necessary if pure Cu3As and NiAs2 were used as standards. These included chemically analyzed α-domeykite and rammelsbergite, the latter containing 0.6 percent Co. With the exception of cobalt in the rammelsbergite the standards constituted stoichiometric and pure compounds. Using a 2 μ beam, the CuKα, NiKα, and AsKα spectra were analysed.
Fig.2. "Mohawkite" and "keweenawite" samples which have been analyzed. Koenig's (1960) bulk analyses include "mohawkite" (M) and "keweenawite" (K). Analyses a to i appear in Table 1. Table 1. Electron Probe Microanalyses of Mohawk Cu-Ni Arsenides
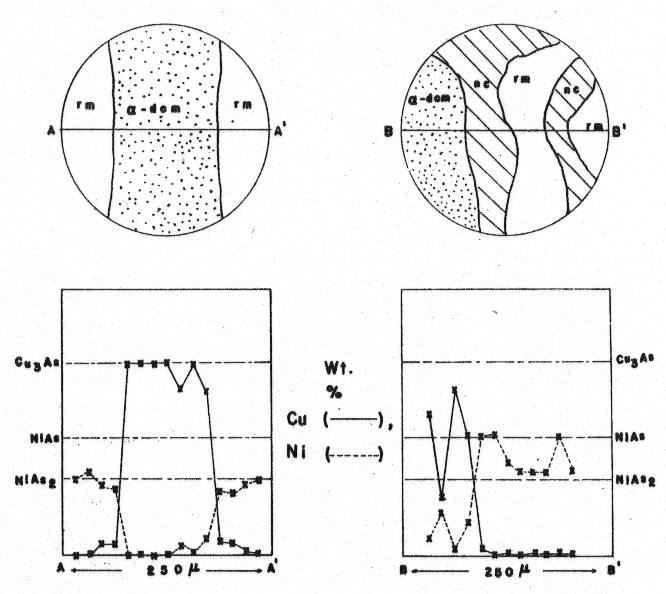
The first set of analyses was based on a polished section of an essentially pure α-domeykite which constituted a portion of the large arsenide masses (Table 1, a to d) and a polished section of "keweenawite" (Table 1, e to i). The results of the analyses are shown in Figure 2. The second set of analyses consisted of traverses across the α-domeykite veinlets which cut the rammelsbergite and niccolite, and these results are shown in Figure 3. Powder diffractographs were obtained for a variety of "keweenawite" and "mohawkite" samples using Ni-filtered Cu radiation and CaF2 as an internal standard. These mixtures all contained variable amounts of rammelsbergite, niccolite, α-domeykite, β-domeykite, and pararammelsbergite. Two typical diffraction spectra of samples from the second set of probe analyses are offered in Table 2. The spacings were indexed according to the cell orientations presented in the ASTM Powder Data File, 1963. A sample consisting of the disequilibrium assemblage pararammelsbergite, niccolite, and α-domeykite was finely ground and heated in a sealed silica tube for seven days at 510±5°C in an attempt to determine the upper limit on the pararammelsbergite inversion temperature. Separation of pararammelsbergite from adjoining niccolite and α-domeykite was prohibitive since the association was intimately mixed. However, the study of the Cu-Ni-As system demonstrates that the presence of copper arsenides and niccolite would have little or no effect on the inversion temperature for the stoichiometric NiAs2 dimorphous pair. The appearance of rammelsbergite after heating (Table 3) shows that the inversion temperature of the dimorphous pair is lowered in the Mohawk material since Yund established this inversion for the pure compounds at 590±10°C at 1 bar by extrapolation in P-T space. Since certain other transition elements and Group V and VI elements are known to strongly influence inversion temperatures of arsenides, even if present to relatively small quantities, it is believed that the presence of cobalt in the Mohawk material has contributed to the lowering of the inversion temperature. Extreme sluggishness of the inversion at lower temperatures prohibited determination of a lower temperature limit. Fig 3. ARL electron microprobe traverses across "keweenawites" interpreted as weight percentages. Crosses falling off the pure composition lines represent finely dispersed mechanical mixtures
A Note on the Cu-Ni-As Subsolidus At 510±5ºC The primary objectives of this experiment were to establish the extent of solid solution of nickel in the copper arsenides and copper in the nickel arsenides, the possible existence of some ternary phase or phases and the fields of coexisting phases. Reactions are relatively rapid at about 500°C and since some of the phases pertinent to the Mohawk disequilibrium assemblage persist to this temperature, this isotherm was chosen. It must be emphasized that the natural assemblages were deposited at lower temperatures and contain binary phases not present in this isothermal system. Although limited solid solubility in an ionic system at a higher temperature indicates even less solubility at some lower temperature, the same inference applied to covalently bonded systems such as the arsenides must be used with caution since electronic band states are sensitive to temperature. We note, however, that there does not appear to exist any isotypic pair in the Cu-As and Ni-As systems even at low temperatures, favoring the inference of probable lack of solid solubility if such is the case at higher temperatures.
Table 2. X-ray Powder Diffractography of "Keweenawite" Mixtures. Cu/Ni radiation, CaF2 internal, errors ±0.003Å
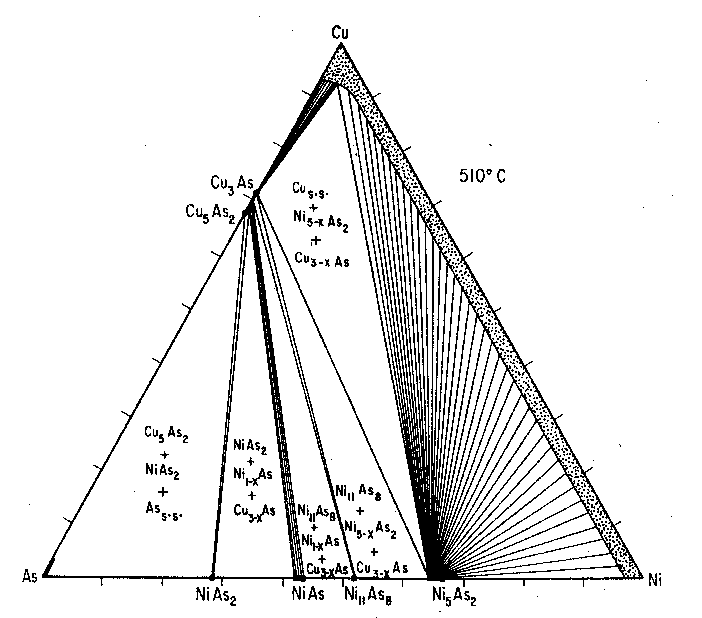
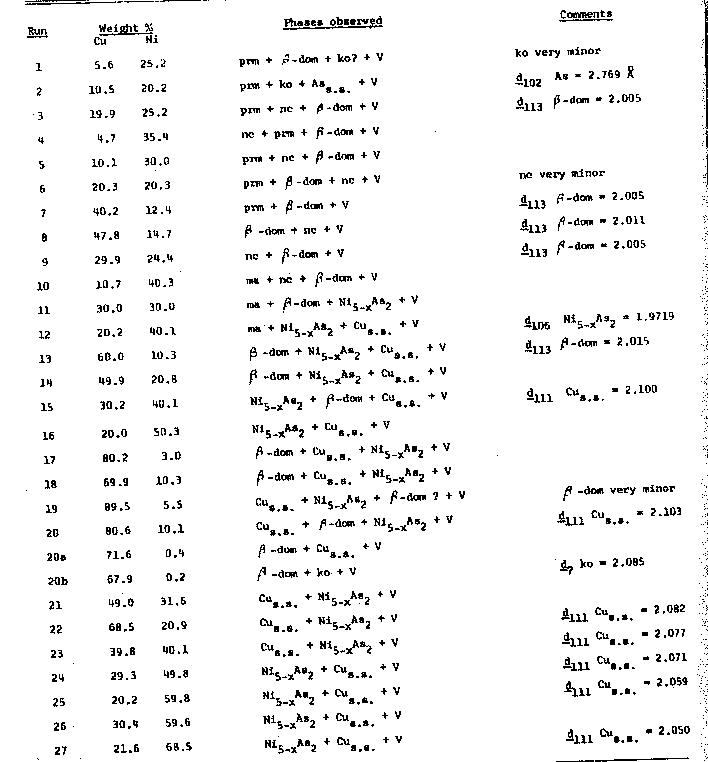
Fig. 4. Interpretation of the Cu-Ni-As condensed system at 510°C. The runs are listed in Table 4. Experimental Nickel containing less than 0.03 percent impurities and copper and arsenic each with less than 0.002 percent impurities were used. All experiments utilized evacuated and sealed silica-glass tubes with a glass rod inserted to tightly pack the charge and to minimize vapor space. The charges were heated at 510±5°C for ten days, reground to assure uniform mixing, sealed and heated for six days more, quenched by dropping in ice water, and then examined optically for inhomogeneities. The materials in all experiments appeared to have reacted rapidly and completely. The phases present were than identified by conventional oscillation powder diffraction techniques, employing CaF2 (a=5.4626± 0.0005Å) as the internal standard.Phases Present. The following solid phases were synthesized in these experiments: copper-nickel-arsenic solid solution (Cu, Ni, As)s.s., β-domeykite (β-dom, Cu 3-xAs), koutekite (ko, Cu5As2), pararammelsbergite (prm, NiAs2), niccolite (nc Ni1±xAs), and maucherite (ma, Ni11As8). The essential features of the Cu-Ni-As condensed system at 510°C are shown in Figure 4; the corresponding charges are listed in Table 4.No ternary phases were encountered throughout this isothermal section. The univariant fields include (As, Cu)s.s.+ko+prm+Vapor (V) 1; ko+β-dom+prm+V; prm+nc+β-dom+V; nc-ma+β-dom+V; ma+Ni5-xAs2+β-dom+V; (Cu, Ni, As)s.s. +Ni5-xAs2+βdom+V. Divariant fields are represented by two thin strips of ncs.s.+β-dom+V, and β-doms.s.+(Cu, As, Ni)s.s.+V; and an extensive (Cu, Ni, As)s.s.+Ni5-xAs2s.s.+V field.Note1 The vapor pressure of arsenic does not exceed 1 Atm. below 604°C. Table 3. X-ray Powder Diffractography of Unheated and Heated Mohawk Pararammelsbergite. Cu/Ni radiation, CaF 2 internal, error ± 0.003Å.
Table 4. Syntheses in the System Cu-Ni-As at 510±5°C
In this study, pararammelsbergite is shown to be the stable dimorph and it is inferred that the presence of coexisting copper arsenides has little or no effect on the inversion temperature. Oscillation diffractographs about the d121 reflection of synthetic pararammelsbergite coexisting with β-domeykite prepared at 510°C as well as d111 of rammelsbergite coexisting with β-domeykite at 650°C show no significant differences in their respective d-spacings when compared with the pure synthetic NiAs2 dimorphs (Table 5).
Table 5, X-ray Powder Diffractography of Synthetic and Monwk Rammelsbergite, Pararammelsbergite, Niccolite, α-Domeykite Assemblages. Cu/Ni radiation, CaF 2 internal, errors ± 0.003 Å
Koutekite, Cu 5As2, is the stable copper arsenide phase in the arsenic-rich portion of the diagram and coexists with arsenic pararammelsbergite and β-domeykite. In the system Cu-Ni-As at 510°C, the koutekite-bearing fields are represented by an extensive ko+Ass.s.+prm+V field and the thin strip kot+β-dom+prm+V. Thus, koutekite does not coexist with niccolite or maucherite. From the NiAs2-Cu5As2 join to the Ni5-xAs2 (x~0.2)-(Cu, Ni, As)s.s. join, β-domeykite is the persistent Cu-As phase. Algodonite (Cu6±xAs) was not encountered since it decomposes at temperatures greater than 300±20°C with composition Cu8As to β-domeykite+Cus.s. and α-domeykite was absent since it decomposes at temperatures greater than 90°C to algodonite and copper deficient β-domeykite, according to Skinner and Luce.It is inferred that nickel plays no significant role in the decomposition and inversion temperatures of the low temperature copper arsenides since essentially no nickel solid solubility in β-domeykite and koutekite at 510°C was detected. Indeed, electron probe analyses of the naturally occurring low temperature coexisting Ni-As and Cu-As phases did not afford any evidence for detectable nickel in the copper arsenides or copper in the nickel arsenides. The lower limit of detectability in the analyses is believed to be 0.1 weight percent. DISCUSSION The presence of cobalt in Mohawk pararammelsbergite does not allow a reliable estimate of the inversion temperature for the naturally occurring pararammelsbergite-rammelsbergite pair. It can be stated that the temperature of emplacement for the nickel diarsenide is certainly below 510°C since the Mohawk pararammelsbergite was experimentally inverted to rammelsbergite at this temperature. Since α-domeykite is the most persistent Cu-As phase in the fissure, temperatures of copper arsenide emplacement were not in excess of 90°C, assuming low pressures in the natural assemblage. These solutions, furthermore, were apparently low in arsenic content since the arsenic-rich phases like paxite (Cu 2As3), novakite (Cu4As3), and (As, Cu)s.s. were not observed. The first two were not observed in the studies of Skinner, Adler, and Mead (1963) in the system Cu-As, which may indicate their slow reaction kinetics at low temperatures, possible metastability, or their stabilization only by impurities present in analyses of natural materials such as Ag.The nickel arsenides, on the other hand, represent phases in the arsenic-rich portion of the Ni-As system. The partial pressure of arsenic was probably too high to allow formation of maucherite (Ni 11As8) and Ni5-xAs2. The reniform appearance of the rammelsbergite as well as the existence of tree-like fractures filled with α-domeykite lead to the conclusion that the nickel arsenides were emplaced earlier in more or less open cavities which were later invaded by copper arsenides. Since the bulk composition of these "keweenawite" mixtures fall within the field Cu3As-NiAs2-NiAs, reaction at the rammelsbergite contact by solutions rich in copper and poor in arsenic leads to the formation of niccolite. In certain instances where small fragments appear wholly engulfed by α-domeykite, reaction led to the complete formation of niccolite which occurs as small blebs in the α-domeykite masses.The rarity of algodonite in association with the great masses of a α-domeykite, phases which can coexist at temperatures below 90°C, indicates that the fluids bore copper and arsenic fractions favoring the precipitation of the stoichiometric Cu 3As phase. It is worthy of note that the algodonite bleb in Table 2 (run d) has composition Cu4.9As, which is close to the arsenic-rich composition for an algodonite in equilibriuim with α-domeykite, according to the phase equilibrium studies of Skinner and Luce (1971) who state the composition Cu5.2As. With the additional source of copper in the adjoining wall-rocks, the precipitation of α-domeykite would lead to the diminution of the arsenic content in the fluids relative to their copper content, with the subsequent precipitation of algodonite and arsenical copper at a later period.An objection may be raised that the copper arsenides were in fact derived from the action of initially arsenic-poor fluids upon the nickel arsenide, and the adjoining copper in the wall rocks. This appears to be quite unlikely since the relative quantities of nickel arsenides encountered during mining operations throughout the Keweenaw Peninsula were insignificant with respect to the copper arsenides. Another observation which militates against this objection is the observed rim of niccolite between the rammelsbergite and α-domeykite; this rim is usually very thin, indicative of only limited reaction between rammelsbergite and the α-domeykite precipitating fluids. ACKNOWLEDGEMENTS Interest in the problem began nine years ago when specimens of the copper and nickel arsenides were personally collected. It became apparent that interpretation of these parageneses was possible only through experiments in the synthetic system. This was realized through the guidance of Drs. G. Kullerud And G. Moh and the use of facilities at the Geophysical Laboratory, Carnegie Institute of Washington, all to whom I offer hearty thanks. Dr. B. J. Skinner kindly supplied a preprint of the recent α-domeykite and algodonite studies prior to publication. Financial assistance was facilitated through a National Science Foundation Predoctoral Fellowship during the years 1963-1965. References BARIAND, P., AND P. HERPIN (1960) Un arseniate de calcium et de magnesium. Bull. Soc. Franc. Mineral. Cristallogr. 83, 118-121. BUTLER, B. S., AND W. S. BURBANK (1929) The copper deposits of Michigan. US. Geol. Surv. Prof. Pap. 144 , 90 JOHAN Z. (1962) Paxite Cu 2As3, a new copper arsenide, Acta. Univ. Carolinae Geol. 1961, 79-86,KOENIG, G A. (1960) On mohawkite, stibio-domeykite, domeykite, algodonite and some artificial copper ansenides. Amer. J. Sci. 4th Series, 10, 439-498. RICHARDS, J. W. (1901) "Moloawkite" Amer. J. Sci, 4th Series, 11, 457-458. SKINNER, B. J., I. ADLER, AND C. MEAD (1963) Phase relations among copper arsenides and their application to geologic thermometry. (abstr) Econ. Geol. 58, 118 ------- and F. D. LUCE (1971) Stabilities and compositions of α-domeykite and algodonite. Econ. Geol., 66, 133-139. WILLIAM, S. A. (1963) Crystals of rammelsbergite and algodonite Amer. Mineral. 48, 421-422. YUND, R. A. (1961) Phase relations in the system NiAs. Econ. Geol, 56, 127.L1295. Manuscript received, December 16, 1970; accepted for publication, January 29, 1971.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||