| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
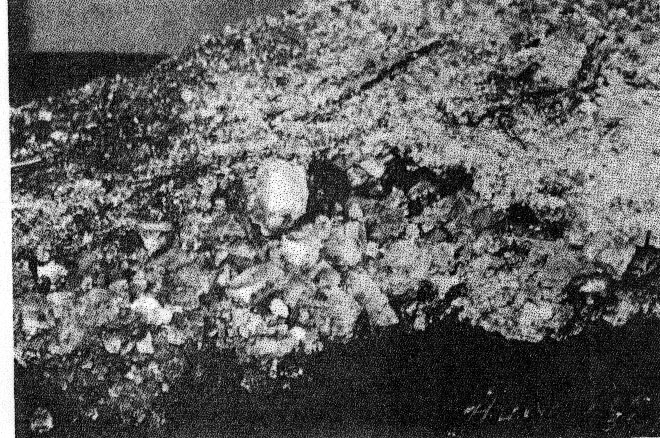
Volume 22, pages 931-933, 1937 AN UNUSUAL OCCURRENCE OF HALITE KIRIL SPIROFF, Michigan College of Mining and Technology, Houghton, Michigan. Recently C. J. McKie, Superintendent of the Quincy Mine, and Professor W. A. Seaman of the Michigan College of Mining and Technology found large quantities of halite at the 85th level of the Quincy Mine, Hancock, Michigan. Since the Quincy is a copper mine located in Keweenawan lava flows far removed from sedimentary strata known to contain halite, and especially since many of the crystals show well-developed octahedral faces, it is believed that the occurrence and the mineral are worthy of description. Sodium and chlorine are commonly found in the deep mine waters1 of the Michigan Copper Mines. Halite has been mentioned2 but once and then only as a few crystals found on the 9th level of the Hecla Mine. The mineral occurs in the form of stalactites, stalagmites and encrustations on the walls as well as on the floor. There are even a few slabs as large as a foot square and two inches thick. The halite varies from coarse crystals of about one inch cubes to very fine thread-like crystals. These fine crystals are so fragile and delicate that even a slight breeze loosens them from the walls and produces what appears to be falling snow. The larger needle-like crystals show square terminations; in other words, they are distorted cubes. Although the large crystals are cubes, many are modified by octahedrons, as shown in Fig. l. They have a salty taste, perfect cubical cleavage, hardness of 3, and a specific gravity of 2.11. The low gravity is probably due to air bubble inclusions. Most of the material is coated with yellow-reddish mine sludge that washes off very readily, leaving the crystals pure white. There are also other encrustations, some red, others green; but not enough of these have been collected for determinative work.
The brown-colored material was analyzed by R. F. Makens as follows: It was washed and dried to constant weight at 110°C. The resulting material consisted of nearly colorless cubic crystals. A qualitative analysis showed traces of Fe+++ Ca++ and SO4-- associated with relatively large amounts of Na+ and Cl-. 0.07% of the original material was found to be insoluble in water. The Na+ was determined quantitatively as NaZn(UO2)3(C2H3O2)9·6H2O according to the directions of H. H. Barber and I. M. Kolthoff,3 and the chloride was determined by precipitating and weighing as silver chloride. The per cent Na+, found as an average of five determinations, was 39.5%; and the per cent Cl-, found as an average of two determinations, was 60.76%. The theoretical percentages of Na+ and Cl- in pure NaCl are 39.32% and 60.68%, respectively. X-ray diffraction patterns4 of the Quincy halite and known sodium chloride were identical. The part of the 85th level where the halite was found in such abundance has not been in use for six years, and the drift is subject to a continuous air current. As the walls, floors, and crevices are covered with halite in a manner similar to the occurrences of calcium carbonate in limestone caves, it is believed that the halite was precipitated by evaporation from waters as they oozed from crevices in the rock or concentrated in small pools on the floor. NOTES 1 Dripping on 55th level north of No. 6 shaft, Quincy Mine
This is the most complete analysis made of the deep water and may be taken as the standard. It is worth noting that calcium and sodium chlorides form 99% of the total salts, and calcium and sodium bromide form three-fourths of the remainder. Lane, A. C. The Keweenaw Series of Michigan: Mich. Geol. Survey, Pub. 6 (Geol. ser. 4), p. 794, 1908. 2 Idem., p. 859. 3 Jour. Am. Chem. Soc., vol. 50, pp. 1625-31, 1928. 4 Fisher, F. Some additional photographs of halite from the Quincy on display at the Seaman Museum - DVB(2001) Photo 1 ( stalagtite is 8 cm long) Photo 2 (largest crystal specimen is 8 cm across)
|