| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
Volume 74, pages 759-763, 1989
Formation rates of calc-silicate minerals deposited inside drillhole casing, Ngatamariki geothermal field, New Zealand
PATRICK R. L. BROWNE
SUE F. COURTNEY
C. PETER WOOD ABSTRACT Chips ejected during the initial openings of Ngatamariki well, NM2, in the Taupo Volcanic Zone, New Zealand, consist of euhedral crystals of wairakite, prehnite, and Fe-rich epidote, plus rare quartz and pyrite. The shapes of some of the ejecta surfaces show clearly that they formed inside the casing and liner slots of the well during the 472 days it was shut in. The calc-silicates were derived from a depth of 1580 to 1600 m where they grew from hot, dilute alkaline chloride water of near-neutral pH and low dissolved CO2 content.
Textural relations and thermodynamic considerations show that, for a time,
wairakite, epidote, and prehnite grew together in apparent equilibrium
from quartz-saturated water with INTRODUCTION Scaling due to mineral precipitation in discharging geothermal wells is usually an unwelcome phenomenon, commonly involving deposition of calcite, aragonite, silica, sulfides, and occasionally magnetite and precious metal precipitates (Arnórsson, 1981; Brown, 1986). Shutin wells are also subject to the effects of mineral deposition, usually of carbonates or sulfates, and casing corrosion. In this paper we report the occurrence of a casing scale composed of a calc-silicate assemblage together with minor amounts of quartz and pyrite. Chips with this mineral assemblage were ejected during the first two discharges from well NM2, Ngatamariki field, New Zealand. Surfaces on some of the chips have a smooth "cast" shape, and this clearly shows that they formed inside the slots of the slotted liner and within the casing itself. Their occurrence, therefore, provides estimates of the minimum growth rates of these crystals, as well as information about their depositional conditions in a hydrothermal environment. THE NGATAMARIKI GEOTHERMAL FIELD The Ngatamariki geothermal field is located 13 km northeast of Wairakei in the Taupo Volcanic Zone of New Zealand (Fig. 1). Four wells, drilled to about 2400 m, penetrated a sequence of calc-alkaline Quaternary rocks, mostly silicic pyroclastic deposits but also andesite lavas and breccias; one well (NM4) reached a diorite surrounded by hornfelsic rocks. Well NM2 was completed to a depth of 2404 m on June 3, 1985; it has production (solid) casing to 888 m, and slotted liner from there to the well bottom. However, because of environmental requirements, its first discharge did not occur until September 18, 1986, i.e., 472 days after well completion; a second discharge occurred several days later. On both occasions the well produced large amounts (perhaps several kg) of crystalline ejecta. COMPOSITION OF EJECTA Almost all the ejecta collected (about 1 kg) consists of pale-gray, spongy aggregates of crystals about 0.5 to 2 cm across. Also present are rare, snow-white crystal aggregates and a few rock chips of white, intensely altered silicified volcanic material. Most of the ejected chips are irregular in shape, with very few showing evidence of their having been attached to a surface such as inside a vein; however, about 3% have the smooth cast surface (Fig. 2) described earlier that proves they grew since the well was completed. Although we cannot prove so, we think it likely that at least some of the non-cast-shaped chips also grew into open cavities inside the casing.
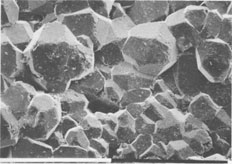
The few white chips ejected consist almost entirely of wairakite, but some of the pale-gray ones also contain prehnite and epidote; tiny (30-µm-long) euhedral quartz and large (about 1-mm-diameter) pyrite crystals are present in some samples. Neither calcite nor anhydrite was seen. Scanning electron microscopy (with EDAX attachment) and X-ray diffractometry aided the petrographic examination, which confirmed the identity of the minerals and showed that the epidote present is an Fe-rich variety and that prehnite and wairakite are nearly stoichiometric. The first mineral to deposit (and survive) was wairakite (Fig. 3), which typically forms euhedral crystals. Those known to have formed inside the well casing are mostly 0.27 to 0.33 mm in diameter, but some chips contain wairakite crystals that average about 0.6 mm in diameter and occasionally are as large as 1.5 mm. Some crystals show pitting that may be due to either local dissolution or, more likely because of their angular shape, to impacts between crystals or with the liner as the ejecta discharged. Prehnite started growing after wairakite, but both phases then appeared to grow concurrently; euhedral prehnite crystals are locally intergrown with wairakite (Fig. 4) and have (101) faces between 0.10 and 0.25 mm long. However, prehnite crystals differ in size more than those of wairakite. Delicate needles of euhedral quartz and more abundant epidote also occur and appear to have grown concurrently with, or after, prehnite. Epidote is pale green, and crystals are typically about 100 µm by 6 µm in size; many show crystal-face terminations, but others have been fractured, presumably during their ejection. WELL DATA Geology of reservoir rocks The geology of well NM2 is imperfectly known because drill cuttings were not returned to the surface from below 1350 m. However, 2- to 3-m-long cores taken at intervals of about 200 m show that the sequence penetrated is dominated by rhyolitic ignimbrites, with minor andesite breccias present near 950 m and another andesitic formation at about 1700- to 2000-m depth. Depth of origin of ejecta The mineral assemblage of the ejecta is very similar to the assemblage of drusy minerals present in cavities in core from 1582 to 1586 m; this assemblage was not found elsewhere. The vertical extent through which prehnite occurs in the host rocks is unknown, but it is absent in both the next shallowest core (1416 m) and the one below (1786 m). Further, it is likely that the ejecta come from, or near to, a permeable zone in the well. Such a zone has been identified at 1580 to 1600 m although this depth is not the main source of fluid production (Gas and Geothermal Trading Group, New Zealand Ministry of Energy). Hydrothermal minerals present in reservoir rocks Wairakite is common in cores and cuttings recovered from between about 800 and 1586 m in well NM2 but was not seen in deeper cores; epidote is present in all cores taken from below 955-m depth. Prehnite occurs only in core recovered from 1582 to 1586 m. The host rock at this depth is a hard ignimbritic breccia whose original phenocrysts comprised plagioclase (andesine) and magnetite; these occurred in a vitroclastic matrix now altered to a fine-grained assemblage of quartz, albite, chlorite, illite, pyrite, and leucoxene. However, primary plagioclase was replaced entirely by adularia, then illite, or else by wairakite and epidote. Filled veins of wairakite and quartz cut the rock, and coarsely crystalline (2-3 mm) wairakite coats surfaces of what were once open veins filled with fluid. The core from a depth of 1582-1586 m also contains rounded cavities, probably formed where pumice clasts were removed, that are partly filled with hydrothermal minerals; these form an assemblage dominated by wairakite, which grew first but was then accompanied by finely prismatic, brown epidote. Later still, after wairakite ceased to grow, minor prehnite crystallized together with epidote. Well conditions The well had a fast heating rate at the depths from which the ejecta are believed to derive (1580-1600 m). The saturation vapor pressure of a steam-pure water mixture at the maximum downhole temperature is above the hydrostatic well pressure so that fluid at 1600-m depth is entirely in the liquid phase. The chemistry of fluid discharged from well NM2 is proprietary information not yet released, but it is probable that the fluid from which the scale deposited was a dilute alkaline chloride liquid of near-neutral pH; fluids of this type discharge from nearby geothermal fields (Wairakei, Ohaaki, Rotokawa and Orakeikorako; Fig. 1), and this is consistent with the observed mineral assemblage. CALC-SILICATE MINERAL GROWING CONDITIONS The environment in which the crystals grew was obviously open space filled with liquid of dilute alkaline chloride type (i.e., nonboiling). The morphology of some ejected chips clearly shows that they deposited inside the slotted liner of the wells; other chips either also formed inside the slotted liner or outside in cavities within the host rock. The occurrence of calc-silicates as scale and in the core from 1580 to 1582 m shows that the liquid from which they deposited was low in dissolved carbonate (<0.3 mol% CO2; Giggenbach, 1984) and sulfate species, since carbonate and sulfate minerals are absent (Browne and Ellis, 1970). The maximum growth time possible for crystals in chips with liner cast surface (and perhaps some others) is 472 days, i.e., the period between the well completion and its initial discharge. However, it is unlikely that the calcsilicates started growing immediately upon completion of the well since it needed to heat up first. The sequence wairakite prehnite - epidote is that usually observed for the distribution of calc-silicates with increasing temperature in the reservoir rocks of New Zealand geothermal fields; these three minerals first appear at temperatures of about 210, 220 and 250°C respectively (Browne, 1984). Assuming that these minerals started forming when these temperatures were reached in the well, then the maximum growing time for wairakite and prehnite is still almost the full 472 days; epidote may have started crystallizing only 10 days after well completion. We do not know if unstable phase(s), e.g., laumontite, formed as the well heated and were then destroyed, but there is no textural evidence of this, and it seems unlikely in view of the very fast heating rate. Observation of chip textures shows that wairakite was indeed the first mineral to precipitate, followed by prehnite and epidote. All three grew concurrently, but, typically, the wairakite crystals grew to about 0.3 mm before being stopped from doing so by space limitations, whereupon new wairakite crystals seeded and grew on top of the older ones.
The minimum growth rates for the three calc-silicate minerals formed inside the slotted liner can therefore be estimated as follows: wairakite crystals (pseudocubic), about 0.30 mm in diameter, formed in 471 days or at the approximate minimum rate of 190 µm3 per day. Prehnite crystals grew at the approximate minimum rate of 0.2 to 0.5 µm per day in a direction parallel to their 110 faces, and epidote crystals grew at the rate of 0.2 µm per day parallel to their 100 faces. The occurrence of these three calc-silicate minerals together in apparent equilibrium allows further interpretation of their formation conditions using thermodynamically and empirically derived relationships (Bird and Helgeson, 1980, 1981; Bird et al., 1984; Giggenbach, 1981, 1984). Some discrepancies apparent earlier between observations made on calc-silicate mineral occurrences in cores recovered from drillholes and calculations made using thermodynamic data have been successfully explained by recognizing the role played by solid solution. For example, Bird and Helgeson (1980) showed that Fe3+ substituting for Al3+, in clinozoisite decreases the minimum formation temperature of epidote so that it can coexist with adularia. The mineral association epidote + adularia is common in geothermal samples but had been previously precluded on thermodynamic grounds by considering the clinozoisite as an end-member composition.
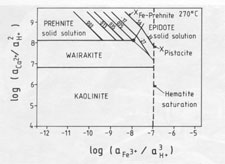
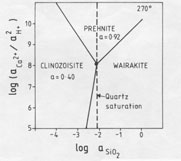
Qualitative analysis of epidote and prehnite by energy-dispersive (EDAX) attachment to the electron microscope shows the epidote of the NM2 scale ejecta as Fe-rich, but Fe was not detected in the prehnite. However, it is likely that prehnite does indeed contain a small amount of Fe (Bird et al., 1984), and consideration of an activity diagram (Helgeson et al., 1978; P. K. Kakimoto, unpublished thesis) drawn for 270 °C (Fig. 5) shows that where wairakite, epidote, and prehnite are in equilibrium, the epidote has a pistacite (Ca2Fe3Si3O12(OH)) mole fraction of 0.20; this coexists with about 0.08 mole fraction of an Fe end-member of prehnite (Ca2Fe(AlSi3O10)(OH)2) (Kakimoto, 1983; Bird et al., 1984). These values, converted to activities for clinozoisite (aClin) of 0.40 and for prehnite (aPr) of 0.92, are used to depict stability relations in Figure 6. Note that quartz saturation of the fluid has been established since euhedral quartz crystals are a minor phase present in some ejected chips. The deduced water composition at 270 °C, therefore, has a log(aCa2+/aH+) ratio of 8 and a log(aFe3+/aH+)ratio of -8 (from Fig. 5). The absence of K-, Na-, and Mg-bearing phases in the ejected scale shows that the depositing fluid did not have a composition that fell within the stability fields of adularia, albite, or chlorite; hence, log(aK+/aH+) was less than 4.0; log(aNa+/aH+) was below 4.6, and log(aMg2+/a2H+) was less than 4.8. To maintain the supply of constituents necessary to form the quantity of minerals deposited requires that the water at 1600-m depth be circulating and not stagnant. For example, wairakite, epidote, and prehnite contain between 22 and 24 wt% Al2O3, whereas the reservoir fluids in New Zealand geothermal fields contain only 0.34 ppm Al (Ellis and Mahon, 1977). Since other constituents are present in much higher concentrations, the Al flux will limit the rate of authigenic mineral growth here. Assuming all the scale was ejected (which is unlikely) and that the 5 kg of calc-silicates was precipitated in the liner, and, further, that the depositing fluid lost all its Al (which is also unlikely), then an order-of-magnitude calculation indicates that about 3000 m3 of water circulated through this part of the reservoir, i.e., at a rate of about 6.4 m3 per day. Undoubtedly the true figure was greater than this.
ACKNOWLEDGMENTS We thank Dr. Jeff Hedenquist and Dr. Keith Nicholson for their helpful comments, and the Gas and Geothermal Trading Group, New Zealand Ministry of Energy, for permission to publish this paper. The paper was improved by comments of the referees. REFERENCES CITED Arnórsson, S. (1981) Mineral deposition from Iceland geothermal waters: Environmental and utilization problems. Journal of Petroleum Technology, 33, 181-187. Bird, D.K., and Helgeson, H.C. (1980) Chemical interaction of aqueous solutions with epidote-feldspar mineral assemblages in geologic systems: I. Thermodynamic analysis of phase relations in the system CaO-FeO-Fe2O3-Al2O3-SiO2-H2O-CO2. American Journal of Science, 280, 907-941. (1981) Chemical interaction of aqueous solutions with epidote-feldspar mineral assemblages in geologic systems: II. Equilibrium constraints in metamorphic/geothermal processes. American Journal of Science, 281, 576-614. Bird, D.K., Schiffman, P., Elders, W.A., Williams, A.E., and McDowell, S. D. (1984) Calc-silicate mineralization in active geothermal systems. Economic Geology, 79, 671-695. Brown, K.L. (1986) Gold deposition from geothermal discharges in New Zealand. Economic Geology, 81, 979-983. Browne, P.R.L. (1984) Lectures in geothermal geology and petrology, 92 p. United Nations University, Reykjavik. Browne, P.R.L., and Ellis, A.J. (1970) The Ohaki-Broadlands hydrothermal area, New Zealand: Mineralogy and related geochemistry. American Journal of Science, 269, 97-131. Ellis, A.J., and Mahon, W.A.J. (1977) Chemistry and geothermal systems, 392 p. Academic Press, New York. Giggenbach, W.F. (1981) Geothermal mineral equilibria. Geochimica et Cosmochimica Acta, 45, 393-410. ________ (1984) Mass transfer in hydrothermal alteration systems-A conceptual approach. Geochimica et Cosmochimica Acta, 48, 2693-2711. Helgeson, H.C., Delaney, J.M., Nesbitt, H.W., and Bird, D.K. (1978) Summary and critique of the thermodynamic properties of rock-forming minerals. American Journal of Science, 278-A, 229 p. Kakimoto, P.K. (1983) Hydrothermal alteration and fluid-rock interaction in the TH3 and THM1 drillholes, Tauhara geothermal field, New Zealand. M.Phil. thesis, University of Auckland, Auckland, New Zealand.
MANUSCRIPT RECEIVED JULY 20, 1988
|