| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
Volume 24, pages 677-680, 1939
C. J. KSANDA, Geophysical Laboratory, Carnegie Institution of Washington, and E. P. HENDERSON,* U. S. National Museum, Washington, D. C. The first good evidence of diamond in a known meteorite was published in 1888, by Jerofejev and Lacbinov. 1 In a stony meteorite which fell in September 1886, near Novo-Urei, they found grayish grains which were regarded as diamonds. Kunz2 was able to procure a small piece of the same meteorite and substantiated their conclusion.
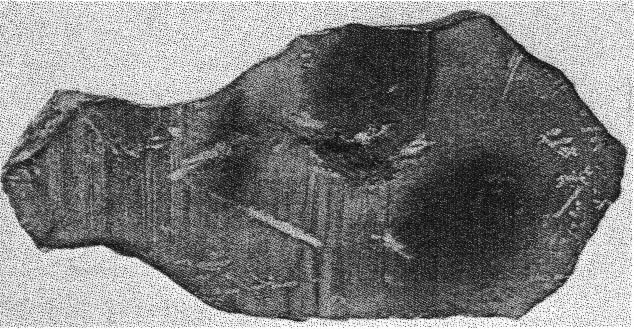
FIG. 1. Section of Canyon Diablo meteorite showing the area in the center of the meteoritic iron from which the diamonds were removed. In 1891 Foote3 reported hard particles in the Canyon Diablo iron. Much. subsequent work in which the mode of occurrence and general crystallographic appearance of these grains are described, is reviewed by Farrington.4 Recently small black grains embedded in a slice from a Canyon Diablo iron have been identified by means of the x-ray powder spectrum method and by microscopic examination as diamonds. The specimen containing these diamonds was purchased by the U. S. National Museum from H. H. Nininger, Denver, Colorado, who while cutting the specimen found small dark inclusions that resisted the saw, and assumed that they were diamonds. The section of iron shown in Fig. 1 is 115 mm. long, 55 mm. wide at its widest part, and weighs 411.7 grams. The specimen was not further polished in the U. S. National Museum laboratories because to do so would probably grind away several of the small diamonds visible to the naked eye.
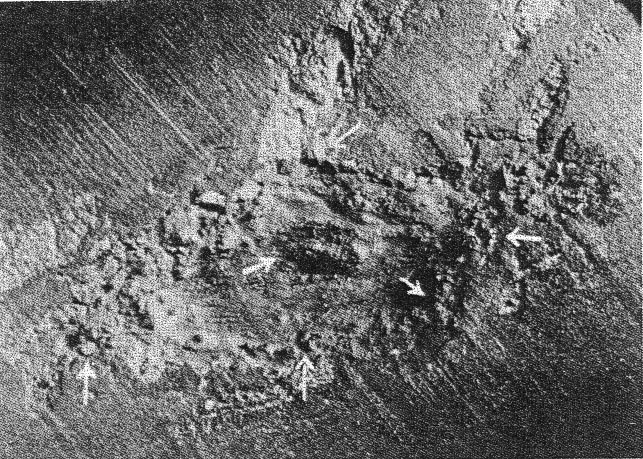
FIG. 2. The cavity enlarged 6X, containing black diamonds, showing larger individuals lining the inner wall, securely embedded in graphite. Arrow points indicate the location of larger grains of black diamonds partly excavated. The diamonds occur associated with some graphitic material within a troilite area. Immediately surrounding the troilite is a series of irregular areas of schreibersite. A wax dam was constructed around the area and the diamonds were freed by repeated treatments of nitric and hydrochloric acid. The insoluble residue was washed out and saved. This residue was slightly contaminated with some organic matter as the acid reacted with the wax dam. The black grains were repeatedly boiled with mixed acids and the acid soluble portion decanted off and rejected. About 50 individual black grains were recovered whose sizes vary from 0.1 to 0.6 mm. The cavity made by the acid (see Fig. 2) is lined with larger individuals securely embedded in the graphite and troilite. Several of these black grains were removed by working them out with a needle point. The largest was 0.9 mm. in diameter. The grains resemble the black diamond, or carbonado, of commerce. Under the microscope they are black, and as recovered are porous, and therefore seem to consist of minute individuals. A rude layering appears in the larger grains. They are more easily broken down than ordinary black diamond.5 Grains rubbed between two pieces of ordinary black diamond did not produce any scratches visible with the microscope. X-ray analysis. Crushed particles were mounted on a fine thread by means of a film of vacuum grease and a photograph was taken with Cu-K radiation in a circular camera of an effective radius of 57.2 mm. The specimen was rotated uniformly during the exposure. The results of the x-ray analysis are given in Table 1. The diffraction pattern shows only the lines theoretically possible within the angle of reflection with Cu radiation for the cubic lattice of diamond. The systematically absent reflections are characteristic of the face-centered lattice type of diamond. The unit cell dimension, do=3.557, calculated from spacings d/n is in agreement with previously published data. 6TABLE 1 Planar spacings and relative intensities of the x-ray diffraction lines of diamond from a rotation powder spectrum taken with filtered Cu-K radiation. Estimated intensities of the diffraction lines are based on a scale of ten, where ten represents the intensity of the strongest line.
ao=3.557 X-ray powder photographs of the graphitic mass were also taken. The resulting diffraction pattern shows a large number of lines, 7 not all of which could be identified. Of the 16 lines measured on the film, only nine are identical in position and intensities with the first nine lines of the known graphite structure.8 No lines characteristic of diamond were found on these films.Optical properties. Under the microscope, in a melt of sulfur and selenium, most crushed grains were not transparent except in irregular patches at thin edges, where they were isotropic; n for orange colored light is greater than 2.37 and for yellow light about 2.42. 9* Published by permission of the Secretary of the Smithsonian Institution. 1 Jerofejev, M., and Lachinov, P., Zapiski Mineralogicheskoe Obshchestvo, Leningrad, (2) 24, 263-294 (1888). 2 Kunz, G. F., Science, 11, 118-119 (1888). 3 Foote, A. E., A new locality for meteoric iron with a preliminary notice of the discovery of diamonds in the iron: Am. J. Sci. (3), 42, 413-417 (1891). 4 Farrington, O. C., Meteorites; their Structure, Composition and Terrestrial Relations. By author. Chicago, 1915. 5 Black diamonds (or carbonado) such as those used for industrial purposes consist usually of a very compact mixture of gray to black and translucent white particles in various proportions, intimately intergrown. The individual crystals are of microscopic size, and the structure when fractured is fine-grained. 6 Wyckoff, R. W. G., The Structure of Crystals, 2nd Ed., Chemical Catalog Co., Inc., New York, 1931. 7 The specimen appeared slightly altered by previous treatment and etching of the cavity with acid. 8 See, for example, Hofmann, U., and Wilm, D., Zeits. f. Elektrochem., 42, 504-522 (1936). 9 The optical properties were determined by Dr. H. E. Merwin, and his aid in this investigation is gratefully acknowledged.
|