| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
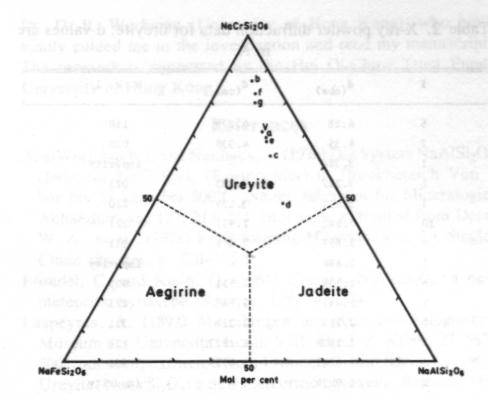
Volume 69, pages 1180-1183. 1984 A terrestrial source of ureyite CHIU MEI OU YANG Department of Geography & Geology University of Hong Kong Pokfulam Roach Hong Kong Abstract Ureyite, a chromium sodium pyroxene of the jadeite group, has been detected in four samples of opaque bright green to dark green Burmese jade. It is a fine-grained fibrous aggregate, intergrown with amphibole. chromite and other minerals. The specific gravity is 3.51-3.52 (obs.) and density 3.55 g/cm 3 (calc.). It has very strong pleochroism (yellow-green) and is biaxial (+) with 2V= 70° (obs.). a = 1 .722, b= 1.734, g = 1.745. The Mossbauer spectrum shows that iron is trivalent (ferric) only. Unit cell parameters calculated from X-ray powder diffractometer data are a = 9.533, b = 8.678. c = 5.265Å, b= 107.49°. Electron microprobe analyses show that it is complex in chemical composition and rich in Al and Fe. The formula for ureyite can be expressed as (Na0.93,Ca0.02)0.95(Cr0.95Al0.17Fe0.10Mg0.03)0.99Si2.02O6.Introduction Ureyite is a sodium chromium pyroxene (NaCrSi 2O6). It has up to now been known in nature only as a rare accessory constituent of some iron meteorites. It was first described (and named kosmochlor) by Laspeyres (1897). It was later investigated by Frondel and Klein (1965) in iron meteorites and they gave the mineral the name ureyite. after Professor N. C. Urey, in place of kosmochlor. Ureyite has been synthesized in the laboratory by several mineralogists. On the basis of unpublished studies D. V. Manson (1979) suggested that the emerald green mineral which composed the Maw-sit-sit jade from Burma might have the same composition as ureyite, but no definite evidence was offered.Recently. ureyite has been identified in four samples of opaque dark green to bright green Burmese jade, and a series of mineralogical studies of this terrestrial ureyite are now reported. Specimen description The ureyite samples were obtained in the jade market of Hong Kong. They are opaque, dark green to bright green Burmese jade. All are compact polycrystalline aggregates, with grain size between 0.03 mm and 2 mm. Sample A is composed mainly of ureyite. A small amount of chromite was detected by microscope examination; this was easily separated using a Frantz isodynamic magnetic separator. The total weight of sample A was 2 g.Sample B consisted of two pieces. each about 10 x 10 x 2 mm and each composed of about 70-80% ureyite and minor amounts of chromite and an amphibole mineral. Most of the ureyite in sample B is in the form of tiny fibers, between 0.05 and 0.1 mm in length, intergrown with the amphibole mineral. Therefore, it was very difficult to separate the ureyite from the amphibole.Sample C is a large sample of emerald green jade weighing about 0.5 kg. It is composed of 50% ureyite, about 30-40% yellow amphibole and a small amount of jadeite and chlorite. Sample D measures about 40 x 40 x 3 mm. It is composed of 50-60% ureyite. 301% amphibole and a small amount of jadeite and albite. The minerals in all of these samples have been identified by microscope examination of thin sections but only sample A was used for the X-ray analysis, the electron-microprobe analysis and the Mössbauer analysis. Chemistry The scanning electron microprobe was used to determine the chemical composition of individual grains of ureyite from the polished thin sections. For the electron microprobe analysis, a JCX A 733WDS with an operating voltage of 15 kV and a beam current of 2 x 10-2 ma were used. The data were corrected by a computer program using Bence-Albee factors. The standards used in the analyses were jadeite for Na and Al; orthoclase for K and Si; glass for Ca. Mg; titanioferrite for Ti; pyrolusite for Mn; chromite for Cr and Fe.The chemical compositions of seven grains of ureyite (Table 1) show an increase of chromium content with a decrease of iron and aluminium. This indicates that they may belong to the NaCrSi 2O6-NaFeSi2O6 and NaCrSi2O6--NaAlSi2O6 isomorphous series. These series, which may not be continuous (see Abs-Wurmbach and Neuhaus, 1976), have not previously been found in natural terrestrial occurrences of clinopyroxene group minerals.Figure 1 shows the relationship between aegirine (NaFe 3+Si2O6). Jadeite (NaAlSi2O6) and ureyite (NaCrSi2O6). As plotted in Figure 1, the results for the seven grains analysed all fall into the chemical field of ureyite. The plotted compositions are more or less on a line which passes through the apex (NaCrSi2O6) and b lies at Ur86 (i.e., 86 mol.% NaCrSi2O6). According to Abs-Wurmbach and Neuhaus (1976), Ur86 is the critical value at which the jadeite-ureyite solid solution has the richest ureyite component at 880°C and 1 kbar pressure. Further research on this point would be worthwhile.The average of the seven microprobe analyses of the ureyite in the Burmese jade gives the following formula: (Na0.93,Ca0.02)0.95(Cr0.95Al0.17Fe0.10Mg0.03)0.99Si2.02O6. NaCrSi2O6 constitutes 70% of the total quantity. Compared with the chemical composition of ureyite in meteorites this ureyite contains appreciable Al and Fe. Unit cell parameters The X-ray powder diffraction pattern (Table 2) was obtained with a D6C diffractometer, with CuKa radiation, NaCl as internal standard, 2° per minute scanning speed, an operating voltage of 40 kV and current of 15 mA. The unit cell parameters determined by the least squares method are a = 9 .533(3), b = 8.678(2), c = 5.265(2)Å and b = 107.49±0.002°.Physical properties The mineral occurs both as short prismatic crystals with length up to 2 mm and as aggregates of tiny fibers (Fig. 2). It has perfect cleavage in two directions parallel to {110} intersecting in 85º or 95°, and a pronounced parting parallel to {001}. The specific gravity is 3.55 (calculated from the unit cell parameters); 3.52 (measured by the micro-heavy liquid method) or 3.51 (measured by the microhydrostatic method).Optical properties The refractive indices vary with chemical composition. For monochromatic light (Na) they are a = 1 .71-1.73, b= 1.72-1.74, g = 1.73-1.75. For sample A they are a = 1.722, b= 1.734, g = 1.745. The mineral is strongly pleochroic, with a yellow, b blue-green, g bright emerald green. It is optically negative with 2V = 70° (measured on the universal stage); the extinction angle a: c = 28 - 30°. These refractive indices are lower than meteoritic and synthetic ureyite. It is suggested that this may due to substitution of Al.
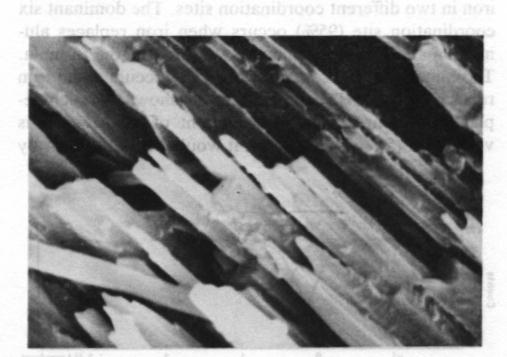
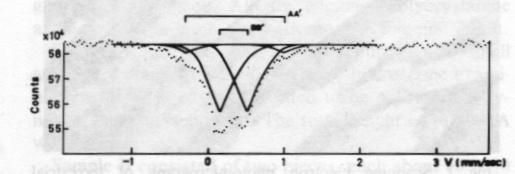
Fig. 2. Scanning electron photomicrograph of terrestrial ureyite (magnification 10000x). The Mössbauer spectrum of the ureyite is shown in Figure 3 and Table 3. The curve was computer-fitted by the least-square method to the standard Lorentian line shape. The Mössbauer results show only trivalent (ferric) iron in two different coordination sites. The dominant six coordination site (95%) occurs when iron replaces aluminum and this replacement is indicated in the formula. The minor four coordination site (5%) occurs when iron replaces silicon. The formula given shows no such replacement, but because the amount of replacement is very small (5% of total Fe 3+), it would be allowed for by the experimental analysis when the expected standard deviation in the result of the electron microprobe analysis is considered.Paragenesis All the four ureyite-bearing samples are polycrystalline aggregates showing granular and/or fibrous texture. They contain 50-95% ureyite, with exsolved jadeite in some cases. The remainder consists of chromite and/or amphibole group minerals, and chlorite. Two habits of ureyite can be observed. One is a short prismatic form up to 2 mm in length, which shows an exsolution texture with jadeite. This suggests that at very high temperatures/ pressures ureyite and jadeite mix more completely together; while at low temperatures/pressures, they tend to unmix. This finding is quite similar to that of Abs-Wurmbach and Neuhaus (1976). The other habit is an aggregate of tiny fibres which can be seen from the extinction pattern to have replaced original grains. Wavy extinction is quite common indicating that the ureyite was subjected to deformational forces during its formation.The process responsible for the paragenetic relation between the ureyite and the chromite is still under investigation. The texture of the chromite gives some hint that it may be the result of alteration of a Cr-rich mineral which has been largely replaced by ureyite.
Fig. 3. Mössbauer spectrum of ureyite
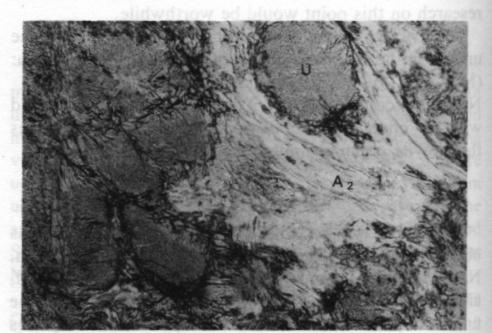
Fig. 4. Plane polarized 35x. U - ureyite; A 1 - amphibole; A2 - amphibole.The relationshp between the ureyite and the amphibole group mineral is clearer. Microscopic studies show that at least two generations of amphibole group minerals are present, as shown in Figure 4. One is a coarsely fibrous form, yellowish green in color and strongly pleochroic, which is found surrounding the ureyite. This amphibole is the result of direct alteration of the ureyite; the replacement phenomena can be observed very easily. Electron microprobe analyses show that it is chromium-bearing sodium amphibole. The other is a finely fibrous form, white to pale green in color and weakly pleochroic, forming tiny veins intruding or enclosing the other amphibole. It seems to be a tremolite-actinolite-series amphibole. However, the chemical composition of these amphibole group minerals is still under investigation. Acknowledgments I would like to express my sincere gratitude to Professor Zhi Zhung Peng (Beijing Graduate School, Wuhan Geological College), Professor Da-Nian Yeh (Institute of Geology Academia Sinica), Professor R. A. Howie (King's College, London), Professor Dorian C. W. Smith (The University of Alberta, Canada), Dr. D. R. Workman (University of Hong Kong), who have kindly guided me in the investigation and read my manuscript. The research is supported by the Hui Oi-Chow Trust Fund,University of Hong Kong.References Abs-Wurmbach, I. and Neuhaus, A. (1976) Das System NaAlSi2O6 (Jaderite)-NaCrSi2O6 (Kosmochlor) in Druckbereich Von 1 bar bis 25 kbar bei 800°C. Neues Jahrbuch fur Mineralogie, Abbandlungan, 127, 213-241. (not seen; extracted from Deer, W. A., et al., (1978) Rockforming Minerals, Vol. 2A Single Chain silicates, p. 520-525.)Frondel, C. and Klein, C. (1965) Ureyite, NaCrSi 2O6: a new meteoritic pyroxene. Science, 149, 742-744.Laspeyres, A. (1897) Mitteilungen aus dem mineralogischen Museum der Universitat Bonn. VIII. Theil. Z. Krist., 27, 587600. (not seen; extracted from Frondel, C. and Klein, C. (1965) Ureyite, NaCrSi 2O6: a new meteoritic pyroxene. Science, 149, 742-744.)Manson, D. V. (1979) Recent activities in GLA's research department; clarification of composition of Maw-sit-sit. Gems and Gemology, Vol. 16, 217-218.
For tables please see original publication
|