| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
Volume 64, pages 432-435, 1979 Ore minerals in a polymetallic deposit of Primorye, U.S.S.R. V. T. KAZACHENKO, V. M. CHUBAROV, I. M. ROMANENKO The Far East Geological Institute Academy of Sciences of the U.S.S.R. L. N. VIALSOV AND G. V. BASOVA Institute of Ore Deposits, Petrography, Mineralogy and Geochemistry Academy of Sciences of the U.S.S.R.Abstract Mn-metasomatites formed by a complex of rare silicates (bustamite, manganoan diopside, pyroxmangite, rhodonite, pyrosmalite, knebelite, spessartine) are widespread in a polymetallic deposit of South Primorye (Soviet Far East). Pb-Zn ores were formed by replacement of manganous metasomatites. As well as the usual sulfides (galena, sphalerite, pyrrhotite, stibnite, arsenopyrite, chalcopyrite, and others), sulfoantimonites of lead (jamesonite and boulangerite), pyrargyrite, and miargyrite, they contain some very rare minerals, such as meneghinite, dyscrasite, diaphorite, gudmundite, antimony, aurostibite, argentian tetrahedrite, and freibergite. A new sulfoantimonite of lead and silver, AgPb 9Sb6S18.5, a new sulfide of iron and silver, (Fe,Ag)xS, and an unusual (antimonian) variety of free gold, Au0.95Sb0.05, are also found in the ores.The presence of this complex of rare and new minerals of manganous metasomatite and ore indicates the specific conditions of formation of the deposit. Introduction This deposit of South Primorye is in the zone of the main Sikhote-Alin synclinorium and occurs in Early Cretaceous sandstones and siltstones. The deposit consists of veinlike bodies of Mn- and Fe-metasomatites formed by a complex of rare silicates. The Mn-metasomatite body is zoned upward: bustamite + manganoan diopside → pyroxmangite + rhodonite (pyrosmalite) → knebelite. During the formation of the polymetallic mineralization the above-mentioned minerals were replaced by rare hydrous Mn-silicates, namely amphiboles, stilpnomelane, and hisingerite. A zonality is also observed in the distribution of amphiboles, which is caused by the primary composition (zonality) of the manganous metasomatites: calcic ones are observed on lower levels (manganoan actinolites) and calcium-poor ones (dannemorite) on middle and upper levels. The Pb-Zn ores were formed by replacement of manganous metasomatites. As well as the usual sulfides (galena, sphalerite, pyrrhotite, stibnite, arsenopyrite, chalcopyrite, and others), sulfoantimonites of lead (jamesonite and boulangerite), pyrargyrite, and miargyrite, they contain a number of very rare minerals, such as meneghinite, dyscrasite, diaphorite, gudmundite, antimony, argentian tetrahedrite, freibergite, and new minerals which are described below. The minerals were analyzed by JXA-5A microprobe at the laboratory of physico-chemical methods of the Geological Institute of the Far East Science Center, Academy of Sciences of the U.S.S.R. Microprobe analyses of minerals were carried out at 20 kV. Standards used for this analysis were: synthetic chalcostibite (for Cu,Sb,S); synthetic chalcostibite and bismuthinite (S); pyrite (Fe); arsenopyrite (Fe,As); galena (Pb); metallic silver (Ag); ZnSe (Zn); metallic gold (Au). The standard errors of the analyses are within ±5 percent. Concentrations were calculated by the computer program of Afonin et al. (1971). Corrections for absorption, atomic number, and characteristic and continuous fluorescence were used. Reflectance measurements were conducted using silicon, carborundum, and pyrite as standards. X-ray powder diffraction data were obtained with a 57.3mm diameter camera and unfiltered Fe radiation on grains removed from polished sections.
Table I. Powder data of new sulfoantimonite of lead and silver
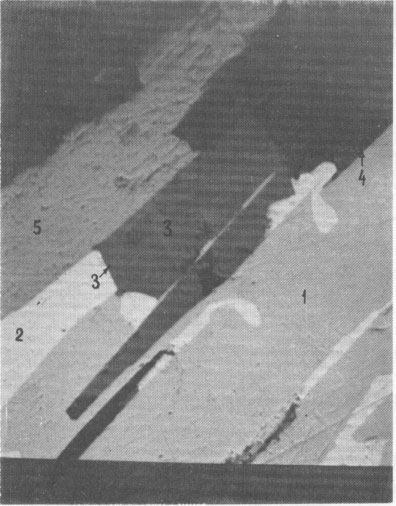
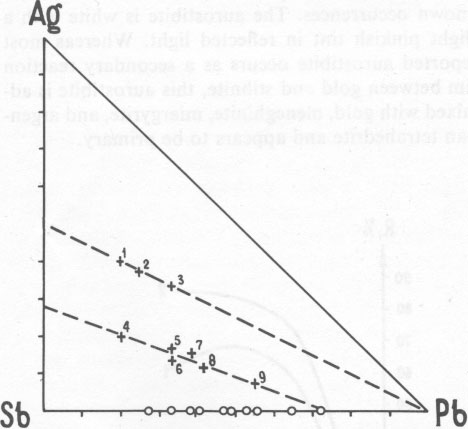
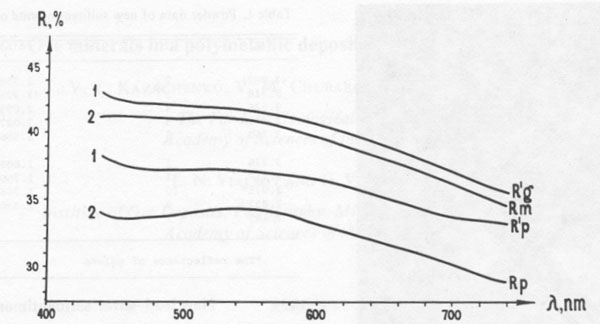
New lead-silver sulfoantimonite A new lead-silver sulfoantimonite was found in the ore. This mineral, which forms fine intergrowths with galena, freibergite, jamesonite, and carbonate (Fig. 1) has been studied by microscope and microprobe in more than twenty grains. In most of these grains it is the main phase. A microprobe analysis of this mineral gave (weight percent): Pb 56.81; Ag 3.45; Sb 22.59; S 18.69; sum 101.54. Recalculation of this analysis to 16 cations yields the formula Ag1.04Pb8.91Sb6.05S18.96. Ideal formulas are AgPb9Sb6S18.5 or Ag2Pb18Sb12S37. This composition (#8 in Fig. 2) lies along a trend of previously known silver-antimony-lead sulfosalts. The powder-diffraction data are given in Table 1. In polished section, this sulfosalt resembles the others of this series. It is especially similar to jamesonite, with which it occurs. Both exhibit approximately the same anisotropism and bireflectance (Fig. 3), and both are characterized by two directions of cleavage - longitudinal perfect and transverse indistinct. This sulfosalt is slightly greenish in comparison to galena but less intense than the green of freibergite. Oriented inclusions of galena are common along the cleavages of the sulfosalt (Fig. 1) but less common in the accompanying jamesonite. Freibergite occurs as rare intergrowths and thin veinlets within the sulfosalt. The freibergite is characterized by the presence of inclusions of a Mn- and Fe-bearing carbonate which is not present in the other phases. It contains 16.0-30.6 weight per cent Ag. New silver-iron sulfide A new sulfide of iron and silver, (Fe,Ag) xS, was found in a carbonate-rich portion of the ore, where it is accompanied by pyrrhotite, sphalerite, antimony, and meneghinite. Microprobe analysis indicates that this mineral contains 10.50 to 22.62 weight percent silver and 47.44 to 54.51 weight percent iron, which is equivalent to Fe0.93Ag0.23S1.00. This composition and an X-ray diffraction pattern and optical properties (Fig. 4) are very similar to those of pyrrhotite and suggest that this phase is an argentian pyrrhotite. This phase tarnished rapidly when exposed to air, with the development of a reddish to violet film.
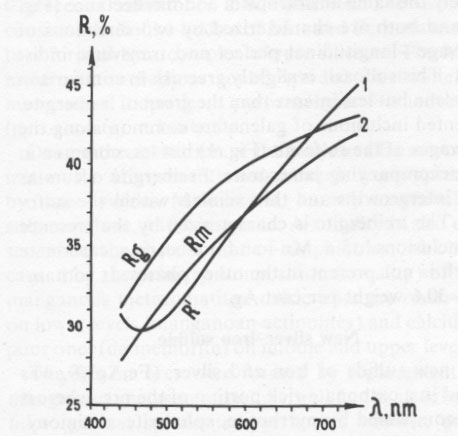
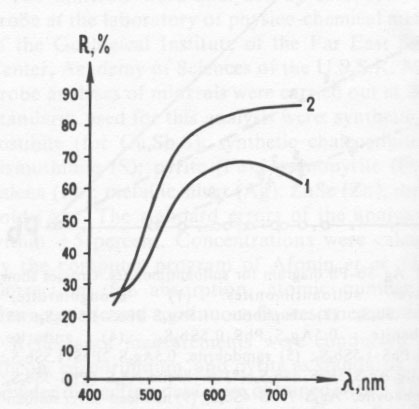
Antimonian gold Antimonian gold has also been found in the carbonate ore. This gold, of composition (weight percent) Au 98.08, Sb 3.17 (Au 0.95Sb0.05), occurs as inclusions with meneghinite, sphalerite, galena, pyrrhotite, miargyrite, and argentian tetrahedrite in carbonate, sometimes as a border along grains of aurostibite. The reflectance of this phase (Fig. 5) is considerably less than that of pure gold and has a maximum at about 650nm.The aurostibite of this deposit has a composition of Au 0.90Sb2.10(Au 41.27, Sb 59.77 weight percent), which is considerably more antimonian than most known occurrences. The aurostibite is white with a slight pinkish tint in reflected light. Whereas most reported aurostibite occurs as a secondary reaction rim between gold and stibnite, this aurostibite is admixed with gold, meneghinite, miargyrite, and argentian tetrahedrite and appears to be primary. Table 2. Powder data of (1) the iron-silver sulfide, and (2) the main reflections of pyrrhotite according to Berry and Thompson (1962, p. 60)
Argentian tetrahedrite (freibergite) The ores of the Primorye Deposit are also characterized by admixtures of argentian tetrahedrite, with up to 30.6 weight percent silver, miargyrite, with 35.8 weight percent silver, and meneghinite, which contains no silver. This assemblage provides information concerning the partitioning of silver among antimony-bearing sulfosalts. Native antimony, dyscrasite, gudmundite, and an unusual variety of arsenopyrite (containing Sb) are rare minerals present in this deposit. The presence of the complex of rare and new minerals in the manganous metasomatite and ore indicates unusual conditions of formation of this deposit.
Acknowledgments The authors are indebted to coworkers of the Far East Geological Institute of the Academy of Sciences of the U.S.S.R., S. A. Korenbaum and S. A. Scheka, and to M. G. Dobrovolskaya of Institute of Ore Deposits, Petrography, Mineralogy and Geochemistry of the U.S.S.R. Academy of Sciences for advice and notes and discussion of this article. References Afonin, V. P., L. A. Perfilyeva and Yu G. Lavrentyev (1971) Program for the computer calculation of element concentration by X-ray fluorescence microanalysis of the various chemical composition specimens. (in Russian) Siberian Geochemical Institute Year Book, 1971, Irkutsk, 398-400. Berry, L. G. and R. M. Thompson (1962) X-ray Powder Data for Ore Minerals: the Peacock Atlas. Geol. Soc. Am. Mem. 85. Chvileva, T. N. (1973) Mineralogical characteristic and diagnostic of sulfoantimonites of lead. (in Russian) Nauka Publishing House, Moscow. Vialsov, L. N. (1973) The reflectance of ore minerals. (in Russian) IGEM, Acad. Nauk SSSR, Moscow. Manuscript received, February 15, 1978; accepted for publication, October 20, 1978.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||