| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
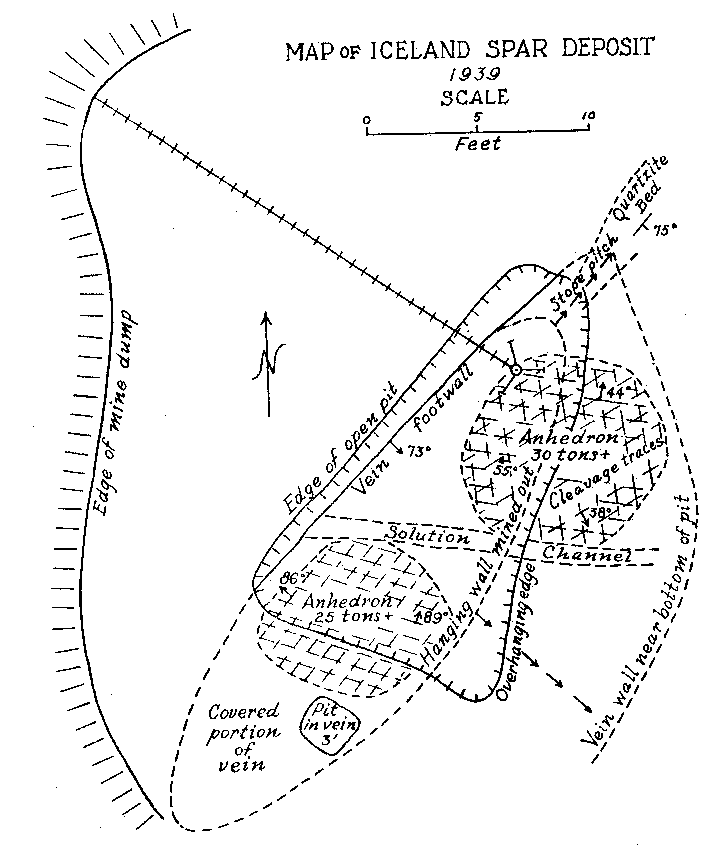
Volume 25, pages 357-367, 1940 ICELAND SPAR IN NEW MEXICO VINCENT C. KELLEY, University of New Mexico, Albuquerque, New Mexico. DISCOVERY AND DEVELOPMENT In 1931 while prospecting the ridge south of the Harding mine, Juan A. Brown of Dixon, New Mexico, unearthed a calcite deposit beneath two or three feet of overburden. Not realizing the character or possible worth of the deposit, its apex was blasted in order to open up and develop the property. The blasting shock undoubtedly ruined some of the near-surface Iceland spar in the deposit, but exposure in this manner revealed material of optical grade. The deposit is located within the confines of the Copper Mountain Mining District in Section 31, T 23 N, R 11 E, Taos County, New Mexico. It is held under the Iceberg claim owned by Juan A. Brown and H. H. Bailey of Dixon, New Mexico. This claim lies parallel to and just south of the Lilac 1 on which is located the Harding mine, producer in the past of trimmed mica, lepidolite, and spodumene. The Iceland spar mine lies about 400 feet southwest of the Harding mine quarry, at an elevation.of 7500 feet. Although discovered as early as 1931, no appreciable development or production was attempted until May of 1939. Since then the deposit has been mined, under lease, in an open pit roughly 15 by 20 feet to a depth of 24 feet. During the last half of 1939 about 850 pounds of optical-grade material was mined, trimmed, and shipped. A considerable portion of this was taken by the Bausch and Lomb Optical Company. The largest single piece of optical-grade Iceland spar obtained from the deposit by the end of 1939 weighed five pounds and eight ounces. At the time of the study by the writer in November, 1939, perfect optical-grade cleavage rhombs of three to four inches on a side were exhibited. Since the completion of this work Johnson1 has published a brief note on the deposit as he saw it a few months after mining began in 1939. GENERAL OCCURRENCE The geology and occurrence of potential and commercial deposits of Iceland spar throughout the world have been briefly described by Hughes.2 From descriptions in this work, it is apparent that most of the Iceland spar has come from hydrothermal deposits in basaltic igneous rocks. Many, like the Helgustadir, Iceland deposit, are in basalt flows where the spar may have an origin similar to zeolites with which the Iceland calcite occurs in primary cavities in the rock or in larger replacement masses and veins. Such occurrences are usually accompanied by considerable alteration, a part of which may be secondary. This type of occurrence, from rather meager information, appears probable for the Cedarville, California deposit, a past producer, and for a non-commercial occurrence near Pyramid Lake, Nevada. A similar occurrence in basic rock, diabase, is reported in the Kenhardt district, northwest Cape Province, South Africa. This deposit has replaced the Iceland source in recent years and has supplied a considerable portion of the European and domestic demand. Except for the Iceland deposits, few detailed studies or descriptions of the paragenesis of Iceland spar veins have been found available by the writer. The Greycliff, Montana, deposits were described by Parsons3 from a report by Dr. S. C. Lind of the U. S. Bureau of Mines. From the descriptions these deposits appear to be rather persistent fissure veins in a country rock of gneiss. This occurrence more nearly resembles the New Mexico deposit from the point of associated country rock. In both of these cases, however, the nature of the country rock has little genetic relationship to the vein material. On the other hand, it appears probable that basaltic flows or intrusives may have some genetic relationship with pure calcium carbonate solutions capable of forming Iceland spar deposits. But until more detailed and specific descriptions are made of Iceland spar deposits from other localities, such as those listed by Hughes4 in Spain, Spitsbergen, Argentina, British Columbia, and other little known occurrences, any statements as to the genetic relationships of Iceland spar are necessarily tentative and without weight. To summarize, however, the known occurrences to date have fallen into two groups: (1) in basaltic igneous rocks and (2) in fissure veins where the nature of the country rock is of little or no importance. It may be of some significance that Iceland spar has not been described or produced from contact metamorphic calcite, nor from cavernous calcite as in the Tri-State deposits. NEW MEXICO OCCURRENCE Geologic Setting. In the vicinity of the Iceland spar deposit and the Harding Mine, the rocks consist of hornblende schists and quartzites. Just5 has mapped and termed these rocks the Hopewell series of Proterozoic (?) age. He found that these rocks were originally a part of a volcanic and sedimentary series which consisted of andesite and basalt extrusives. (Picuris basalts) intercalated with some clastic sediments. The Hopewell series in this area forms a narrow belt of from one-quarter to one mile in width trending about N 70° E.. This belt is part of the south limb of a syncline which plunges westward. The series is the oldest of the Proterozoic (?) mapped in the general area. To the south of the deposit, a distance of about one-quarter of a mile, the schists and quartzites are intruded by the Dixon granite which Just6 has tentatively dated as late Proterozoic (?) (Killarney) age. The Iceland Spar Deposit. The Iceland spar forms a lenticular, pipelike deposit which lies nearly parallel to the schistosity of the Hopewell series. The footwall of the deposit dips S 45° E at 73°. The vein has a length of about 30 feet at the surface and a maximum width of nine and a half feet near the center. Johnson7 states that the deposit appears to plunge to the southwest. Since the deposit was not opened up to the end in this direction, it is difficult to see how this conclusion was reached. At the time of the study by the writer the northeast end was completely exposed and the pitch of the vein in this direction was clearly seen as shown in Fig. 1. The walls of the vein are coarsely brecciated and altered for a width of from one to three feet. The alteration probably extends to a slightly greater distance into the wall than the brecciation. The breccia fragments on the whole consist of angular, altered schistose blocks, one to two feet in diameter. In the northeast end of the breccia sheath are blocks of quartzite derived from the end of a quartzite lens in the schist series. This quartzite bed widens from a foot or so in the northeast end of the vein to 10-15 feet on the top of the low ridge some 50 feet northeastward along the strike. The alteration of the breccia zone adjacent to the vein has produced a softened, brownish to yellowish green mass. Under the microscope the hornblende of the schist is seen to be altered to biotite, golden yellow (bleached) biotite, limonite, and kaolinitic material. The quartzite blocks along the northeast end of the vein have been replaced in patches and veinlets by ankerite which has been later surficially altered in part to limonite. About 50 feet southwest of the vein, the hornblende schist is much altered to epidote. Just8 reports epidotization of the hornblende schists as a contact metamorphic effect near the Dixon granite a short distance to the south. Some bleached epidote occurs in the altered breccia zone which might be taken as part of the wall-rock alteration genetically associated with the calcite deposition. However, it is probable that such epidote was earlier and was incorporated only by chance in the breccia and alteration zone which accompanied the calcite vein formation.
The vein proper is composed almost entirely of exceedingly coarse anhedral calcite. No euhedral calcite is present. Several small solution cavities were encountered in the top of the deposit. These were mostly filled with surficial kaolinitic material. One larger solution cavity or "watercourse" across the vein persists to the mined depth as shown in Fig. 1. A few lentiles or inclusions of highly altered schist were found in the vein. The edge of the vein is quite irregular in detail. Small blunt offshoots of the calcite protrude into the breccia wall. Some of these are still left as isolated patches in the hanging wall. They appear as replacements of individual breccia fragments. A few other minerals occur in the calcite in very minor quantities. Chalcopyrite replaces the calcite in irregular and disseminated veinlets. Associated with the chalcopyrite is a fine, granular, greenish calcite. The chalcopyrite occurs as tetragonal scalenohedrons imbedded in the spar and in the greenish granular calcite. The chalcopyrite is mostly surficially altered to chrysocolla, tenorite, and limonite. The greenish color of the granular calcite is due to chrysocolla and it is possible that the granular calcite is supergene. Calcite. Three types of calcite occur in the deposit. The most abundant type is white calcite: Next in abundance is the clear, colorless calcite from which is culled and trimmed the optical spar. The third type is a banded pink calcite. When the pink calcite was first found it was thought to contain lepidolite because of the similarity in color and the proximity to the lepidolite-bearing pegmatites of the Harding mine. Nearly all gradations between these three calcites occur in the vein. The pink material in the calcite is always in fuzzy, closely spaced bands or layers which are only a fraction of a millimeter thick. The bands are not twin lamellae. They are due to a "salt and pepper" scattering of very small colloidal particles or aggregates of particles, averaging about 0.05 millimeter in size, which are aligned along definite crystallographic planes.
The more common banding is parallel to the rhombohedral cleavage. The only other
banding, although not as common, is parallel to the scalenohedron 21 Cleavage pieces are often pink, banded in one sector and clear in the remainder. The banding terminates irregularly in some specimens although in others it ends evenly along planes such as those of the scalenohedron. Banding parallel to both the rhombohedral and scalenohedral directions in different sectors of a single cleavage piece sometimes occurs. The pink banded material is more closely associated with the Iceland spar type of calcite.
Much of the calcite of all three types is twinned. Much of the otherwise
optical-grade Iceland spar is of no value for this reason. The twins are of two
well known types. Large individual twins occur twinned on c or parallel to
(0001). The most common twinning is polysynthetic parallel to (01 One of the most unusual features of the calcite, noticed principally in the colorless and pink types, is the existence of a perfect conchoidal fracture. This perfect glass-like fracture was found in several pieces on the dumps and was subsequently developed on several occasions in the laboratory. Dana 10 mentions this fracture as "obtained with difficulty" because of the excellent rhombohedral cleavage.In order to discover the impurities in the pink calcite, spectrographic analyses were made of it and the Iceland spar. These analyses were made by Mr. Guy V. Martin, Martin Laboratories, Albuquerque, N. M. Samples of the two calcites and Baker's c. p., calcium carbonate were compared, with results as follows:
t-spectrographically present; less than 0.0005. The results for the pink spar are an average of two samples-one of average pink and another so strongly pigmented as to be almost brown. From these results it is seen that the material pigmenting the pink calcite so strongly is present in only very minute quantities. Both the pink calcite and the Iceland spar are purer than the Baker's c. p., calcium carbonate. Both manganese and magnesium are present in the Iceland spar as well as in the pink spar. Why then is the Iceland spar not colored since both iron and manganese are present? The greatest difference lies in the iron content, and possibly the iron is the principal cause of the pink color. Manganese, however, is commonly thought to give rise to pink color in carbonates such as rhodochrosite. Perhaps a significant difference in the composition of the two calcites is the presence of silicon and aluminum in the pink spar and their absence in the Iceland spar. The explanation put forth partly at the suggestion of Mr. Martin is that the silicon and aluminum in colloidal form tend to localize or retain the manganese and iron pigments in a manner which brings out the color.
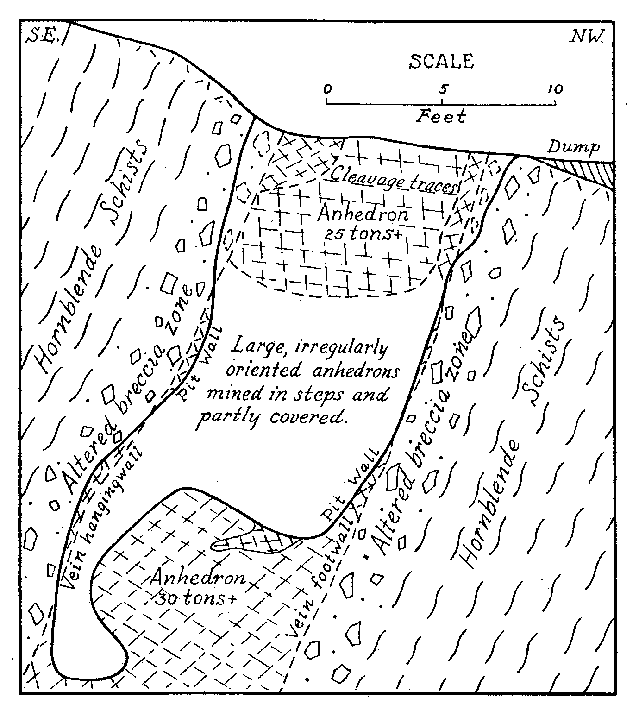
Some banded pieces observed under a binocular show very tiny dendritic growths of the pink material more or less normal to the planes of banding. These are suggestive of the "silicate gardens" produced by soluble compounds absorbed in silicate gels. Pycnometer specific gravity determinations of the Iceland spar and pink banded calcite were made for reference. The following are values obtained at room temperature: Iceland spar ........ ...... ................ 2.716 Pink spar ....... ............... .......... 2.721 Large Crystals. Although the vein as exposed is rather small, some of the crystals are gigantic. Portions of two very large crystals were exposed on the side and bottom of the pit at the time of study by the writer. The larger of these occurred practically at the surface with cleavage traces oriented as shown in Fig. 2. The southwest side of the pit (Fig. 1) coincided with the third rhombohedral cleavage direction which was vertical with strike S 72° E. The top of this crystal had been partly eroded away. The crystal extended into the southwest wall for at least two feet at the top. About six feet of the crystal had been mined away on the northeast side. The more striking anhedral crystal occurred in the bottom of the pit with the c-axis nearly vertical as shown in Fig. 2. This crystal was exposed on three sides including a portion of the underside. Some of the exterior of the crystal had been stripped off by mining. Before the end of 1939 the entire crystal was removed but a very good idea of its size and position can be obtained from Fig. 3. From the dimensions of the remaining crystal at the time of the study it is estimated to have weighed at least thirty tons, or possibly as much as forty tons before it was partially mined. Both of these crystals rank second in size to the large Iceland crystal described by Des Cloizeaux and recorded by Palache. 11Origin. Points to be considered in connection with the genesis of the Iceland spar deposit are: (1) the brecciation zone in the wall rock, (2) the nature of the wall rock alteration, (3) the purity of the calcite, and (4) the pink banding of the calcite. The brecciation is spacially and genetically related to the calcite vein. It is a peripheral zone about the vein and has no extension beyond a few feet in any direction from the vein. The breccia is a mechanical one rather than a replacement breccia. It is not a fault breccia as indicated by Johnson, 12 and little or no slickensiding is present. The volume of the breccia as compared to the size of the vein is large as in many breccia pipes. Thus the channelway for the carbonate solution appears to have been produced by rapidly released gaseous emanations which moved upward from depths along the foliation of the hornblende schists. A small lenticular, pipe-like channel produced by such means would be clogged by large angular blocks torn from the walls. At least locally, the void space might be considerable.The gaseous emanations producing the pipe were probably in part responsible for the alteration of the breccaated wall rock. However, some of the alteration, if not a major portion, was produced by the carbonated solutions which later deposited the calcite. Some supergene kaolinization was effected in the top of the alteration zone. This is whitish in color in contrast to the green coloration of the altered wall rock deeper in the deposit. Because of the difficulty encountered in a mineralogic and petrographic study of the altered rock, spectrographic analyses were made of the material. These investigations indicate rather clearly the hypogene nature of the wall rock alteration. Several analyses were made of typical unaltered hornblende schist adjacent to the walls of the deposit. Several similar analyses were also made of the various stages of altered wall rock. By comparison with the unaltered rock, the altered material showed additions of nickel, barium, bismuth, chromium, and molybdenum. The presence of these elements in the altered rock and their absence in the original wall rock precludes any probability that the alteration was supergene. Was the calcite of the vein deposited by filling of a cavity already present or by replacement of much brecciated material, or by both processes? The deposit shows no characteristics of cavity filling such as crustification, banding, or primary vugs. However, recrystallization could have obliterated such primary structures. Some filling of space in the breccia pipe must have occurred since a pipe so conceived would in all probability contain numerous super-capillary openings. It might be suggested that the very purity of the calcite should indicate an origin by filling of an open cavity or pipe because replacement would contaminate the solution so as to prevent deposition of pure calcite. But this is in effect proposing that host minerals cannot be replaced without leaving some trace in the metasome, or that replacement cannot be complete. If purity of a vein or a mineral be used as a criterion of cavity or fissure filling, then all veins of pure quartz, pure calcite, pure pyrite, etc., must be of such origin. A criterion of this nature would relegate replacement to a minor role in mineral deposition. In spite of purity of the calcite it is believed that replacement was the principal mode of deposition of the vein, although filling of the void space present in the pipe necessarily took place.
The origin of the pink material in the banded calcite is a problem. It appears
to be easily explained as a paragenetic intergrowth. If all of the pink material
were confined to crystallographic planes such as that of the unit rhombohedron
and the scalenohedron (21 By way of conclusion and summary, the deposit is of hydrothermal origin. The mineral channel was formed by vigorous gaseous escape from depths, which formed a lentictilar breccia pipe parallel to the schistosity of the country rocks. Wall rock alteration was initiated at the time of brecciation and continued until the calcite deposition. The calcite deposition was accompanied by replacement of breccia fragments which filled the pipe. That the wall rock alteration and calcite deposition were both hypogene is indicated by such metals as nickel, chromium, and molybdenum in the rock and chalcopyrite veinlets in the calcite. If any usual evidences of fissure filling existed, they were early obliterated by recrystallization and incorporation of smaller crystals into the larger anhedrons. Late hypogene infiltration of colloidal pigmenting impurities gave rise to the banded calcite. NOTES 1 Johnson, J., Harlan, Iceland spar in Taos County, New Mexico: Am Mineral.. 25, 151-152 (1940). 2 Hughes, H. Herbert., Iceland spar and optical fluorite: U. S. Bureau of Mines, Information Circular 6468 (1931). 3 Parsons, Chas. L., Iceland spar in Montana: Science, New Series, 47, 508-509 (1918). 4 Op. cit., pp. 8-12. 5 Just, Evan., Geology and economic features of the pegmatites of Taos and Rio Arriba Counties, N. M.: New Mexico School of Mines, Bull. 13, 21 (1937). 6 Op. cit., p. 24. 7 Op. cit., p. 151. 8 Op. cit., p. 25. 9 Whitelock, Herbert P., Calcites of New York: New York State Museum, Memoir 13, 73,77 (1910). 10 Dana, J. D., System of Mineralogy, 266 (1909). 11 Palache, Charles, The largest crystal: Am. Mineral., 17, 362-363 (1932). 12 Op. cit., p. 152.
|