| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
Volume 10, pages 355-411, 1925 THE PARAGENESIS OF THE GRANITE PEGMATITES OF CENTRAL MAINE KENNETH K. LANDES,* Harvard University SUMMARY A study of the paragenesis of certain pegmatites in central Maine has caused the writer to advance the following ideas which differ somewhat from those usually held on the subject. The essential features of his thesis are: A. Mineralization of the pegmatites was accomplished in successive stages. The minerals deposited during each stage compose a distinct group or class. I. During the first stage alone there was a mass crystallization from the liquid phase. The typical minerals of this class are potash feldspar (usually microcline), quartz, muscovite, biotite, black tourmaline, beryl, garnet, arsenopyrite, and manganapatite. II. This was followed at every deposit studied by a high temperature hydrothermal phase. The volatile constituents of the magma, which played a rather minor part in the first stage now become of supreme importance. During the crystallization of successively lower portions of the magma the "mineralizers" are expelled and work upward, depositing new minerals as they do so. While the magma at hand and at slight depth was crystallizing high temperature conditions prevailed, and the first class of hydrothermal minerals resulted. Typical minerals of this period are: quartz (usually in crystals), cleavelandite, lepidolite, lithia tourmaline, columbite, cassiterite, spodumene, and etched or pocket beryl. III. At Buckfield, and to a very minor degree at Mount Mica and Auburn, an intermediate phase followed during which rare lithium-manganese phosphates were deposited by the ascending solutions. Typical products of this phase are: amblygonite, lithiophilite or triphylite, rhodochrosite, eosphorite or childrenite, fairfieldite, and reddingite. IV. A final hydrothermal phase which was very well developed at Buckfield, Greenwood and parts of Auburn resulted in the deposition of large amounts of cookeite and quartz. At Greenwood and Auburn purple apatite crystals were also formed in abundance. Typical minerals are quartz, cookeite, and apatite. V. Ground water alteration of material already present produced the last class of minerals. The minerals of this type are mainly kaolin and montmorillonite. Locally manganese oxides, dahllite, and francolite were formed. B. The pockets of the Maine pegmatites are largely secondary, produced by dissolving activity of the same ascending solutions which deposited the rare minerals of the second and later classes. INTRODUCTION The granite pegmatites of central Maine have been described in some detail by Bastin.1 The present paper presents the results of a more detailed investigation of two recently opened deposits and some additional facts concerning some of the better known localities. Recent feldspar quarrying operations at Buckfield, in Oxford County, have uncovered a remarkable suite of minerals. Detailed study of some two hundred specimens from this locality now in the Harvard mineralogical laboratories has resulted in the identification of over thirty minerals, a half dozen of which appear in but one or two other localities. This intensive study, coupled with observations in the field, developed the thesis that the minerals were by no means products of one period of crystallization but group naturally into five classes. Although the result of a practically continuous process the classes differ from each other in chemical, physical, and mineralogical character. Only one class, the first, solidified as a magma. The minerals of the main pegmatite, mostly microcline and quartz, belong here. The next three classes represent successive waves of hydrothermal activity from the slowly cooling igneous mass beneath. The solutions were replacive, dissolving the old and depositing the new. At times the former process exceeded the latter, and open spaces or pockets were left, usually lined by drusy crystals. A gradual change in the character of the solutions produced a sequence of minerals within a class. A more radical change resulted in the initiation of a new class. The first of these hydrothermal groups, Class II, is characterized by cleavelandite, low temperature quartz crystals, transparent tourmalines, and many other minerals. Here we find most of the "pocket" minerals prized by the collector. Class III is a rare phase, present to any great extent only at Buckfield, Maine, and Branchville, Connecticut. The unusual phosphates lithiophilite, eosphorite, fairfieldite, and reddingite belong here. The Class IV minerals, chiefly cookeite, quartz, and apatite, are distinctly the result of a relatively late but active mineralization. This phase is developed to a considerable extent in several of the central Maine pegmatites. In Class V are placed the supergene alteration products of the minerals in the preceding classes. Several mineral species appear in more than one class. But when this is true differences in either habit or physical, chemical, and optical properties are found and the mineral may usually be placed as definitely as though different species were involved. With beryl and apatite these differences are indeed striking. This, briefly, is the thesis advanced in the following pages. The locality used for illustration is Buckfield, for many reasons. The collections from here are most complete. The range of minerals is large. The presence of one more class than usual, containing the rare phosphates, makes the deposit of interest. Furthermore, recent quarrying operations have made the interior of the pegmatite accessible for field study. The locality is a new one, practically undescribed in print.2 Briefer descriptions of parallel deposits follow. Greenwood, although completely lacking the minerals of Class III, illustrates the other classes well. The control of structure on the ascending solutions is better shown here than at Buckfield. Mount Mica and Auburn, two old and famous mineral producers, are likewise briefly considered from the standpoint of paragenesis.
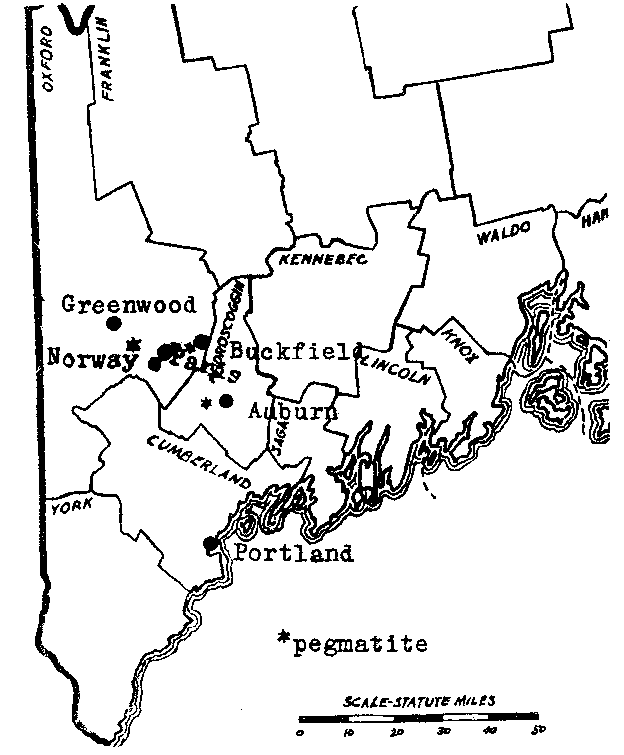
FIG. 1. Southwestern Maine showing location of pegmatite bodies described in this report. LOCATION A map showing the approximate location of the pegmatites discussed is given on Plate I. The village of Buckfield is on the Rangeley division of the Main Central Railroad. A dirt road runs to the eastward to Paris Hill and South Paris, the latter a town on the main line of the Grand Trunk. The Buckfield pegmatite is on this road four miles from Buckfield village, and seven miles from South Paris. A private road at Bennett's farm runs north from the main road three fifths of a mile to the deposit, which caps a rounded, glaciated hill. The pegmatite body has been opened up on the west flank of the hill by quarrying operations of the Maine Feldspar Company.The Greenwood deposit is located on the southwest side of Noyes Mountain in the town of Greenwood, two miles south of the village of that name, and eleven miles north of Norway. A trail leaves the Norway- Greenwood road at the crossroads near the foot of the mountain and climbs up 350 feet to the pegmatite. An old producer of mineral specimens, this deposit was reopened in the summer o f 1923 by the Harvard University Mineralogical Department. The operations of that summer lowered the floor of the quarry some fifteen feet and drove the breast back about ten feet. The quarry is now idle. FIELD AND LABORATORY WORK Active quarrying for feldspar has been going on at the Buckfield quarry since the summer of 1923 and the writer made frequent visits there during the summer and the following spring. In the late summer of 1923 Professors Palache and Larsen visited the quarry just after a large pocket had been revealed and they made an extensive collection. Mr. W. D. Nevel of Andover, Maine, also kept a close watch on the quarrying operations and most of his collections were acquired by the Harvard Museum. All of this material has been available for study. Operations at Noyes Mountain were begun many years ago by Mr. George L. Noyes of Norway, Maine. The minerals he collected were largely preserved and passed into the possession of the Harvard Museum, together with the lease of the property, in 1917. In the summer of 1923 active quarrying was undertaken by the Harvard Mineralogical Department under the supervision of the writer. Loren B. Merrill of Paris, Maine, did most of the actual work of excavation, and gave to it the invaluable experience gained from a lifelong familiarity with similar deposits at Mt. Mica and elsewhere in Oxford County. The bulk of the work was performed in the laboratory. Specimens from both localities, especially Buckfield, have been studied by the use of immersion liquids and the microscope, and by blowpipe and chemical tests. The electromagnet and the Clerici heavy solution were employed to secure pure material for analysis. In some cases the quantities involved were so small that microchemical methods were necessary. Particular attention has been paid to the order of formation and the associations of the minerals, for here lies the clue to their genesis.Eight of the more unusual minerals have been analyzed by Miss Helen Vassar, the departmental chemist. These analyses bring out several points of interest and will be included with the mineral description. ACKNOWLEDGMENTS The writer wishes to express his sincerest thanks for the help rendered throughout the work by Dr. E. S. Larsen, both in the determination of minerals and in the theoretical discussion. Many of the ideas worked out and advanced here have long been held by Dr. Larsen, especially those on the later albitization of pegmatites. Dr. Charles Palache has afforded a constant inspiration throughout the course of this study. Through his aid the field work was made possible, and by his suggestion the study was made. He has not only devoted much of his own time to the study, but has also thrown the resources of the department behind it. BUCKFIELD LOCAL GEOLOGY Study of the local geology at Buckfield is made difficult by an extensive covering of glacial till. Gneiss bounds the pegmatite body on the east and south, granite on the west and north. The former is the country rock of the region while granite is more local in extent. In the hand specimen the biotite gneiss shows parallel bands of biotite with orthoclase and quartz between the bands. Minerals present to a minor extent are apatite and pyrite. The rock is a metamorphosed granite. The granite is light colored and has a fine grained aplitic texture. It is rich in quartz and contains also essential oligoclase, microcline, and muscovite, a little biotite, mostly intergrown with muscovite, and traces of garnet and hornblende. Occasional splotches of black tourmaline are present and it evidently underwent attack by portions of the still liquid magma as quartz encroaches on it extensively. The contact of the granite and gneiss was nowhere found but the unmetamorphosed character of the granite shows that it is much younger than the gneiss. It is probably related magmatically to the pegmatite.PEGMATITE DESCRIPTIONThe pegmatite body caps a low hill and is flat or dips at a low angle to the east. Its areal extent is large, the paced distance from the footwall contact with granite on the west flank of the hill to the gneiss contact on the eastern slope measuring over five hundred feet. At the top of the hill six hundred and fifty feet, apparently all pegmatite, separates gneiss on the south from granite on the north. This dimension may be considerably less to the west, where exposures are lacking. Mining has proceeded on two levels. The upper terrace extends from the surface back into the hill for about a hundred feet, where, due to the natural slope of the hill it has a depth of about thirty-five feet. The breast at this point is forty feet wide. A second cut, fifteen feet below, extends into the hill for a lesser distance. A small pit four hundred feet to the northwest opens up pegmatitic material exceptionally rich in beryl. The mineralogical similarity between this deposit and the much larger one suggests that they are related intrusions. The size of grain of the minerals in the main pegmatite varies greatly. Adjacent to the contact with the country rock the feldspar and quartz anhedrons are no larger than those in the interior of the average granite. Outside the contact phase these minerals appear in masses up to ten feet across. Some examples of this latter size may be seen on the face of the quarry. The minerals of the first class make up the main pegmatite and are rather uniformly distributed, but those of later hydrothermal deposition, belonging to Classes II, III, and IV, are confined to a relatively small zone exposed in the lower workings of the quarry. In opening up this level, the miners first passed through an area of fallen or slumped pockets. Massive feldspar and quartz of the walls were mixed with quartz crystals, some of large size, belonging to a later generation. Surface waters had added a coating of clay to the debris. As operations extended the cut farther into the hill, and correspondingly farther from the surface, undisturbed pockets were encountered. METHOD OF EMPLACEMENT The pegmatite shows various features which indicate that it was an igneous intrusion. In the first place the size of grain is greatly diminished bordering the contact with the country rock, implying a chilled phase about the periphery of the magma. Furthermore, the minerals crystallized in a definite sequence, identical to that in a granite. Lastly, there are inclusions of gneiss in the pegmatite more or less resorbed. The evidence appears to be strongly in favor of deposition by a viscous rather than a highly liquid solution. The presence of a higher percentage of volatile constituents than is customary in a granite resulted in a lower crystallization temperature, which permitted the formation of large crystals and the complete conversion of orthoclase to microcline. But of far greater importance was the post-magmatic activity of these volatile constituents in the formation of later minerals. LATER ACTIVITY It is the thesis of this paper that mineralization of the pegmatite did not cease with the magmatic crystallization just described, but continued for some time, through the agency of ascending solutions. These were expelled by minerals crystallizing from successively lower portions of the igneous mass. In their upward and outward movement the hot solutions dissolved and replaced earlier minerals. Large pockets were formed, pre-existing minerals altered, and new minerals superimposed. The minerals of Classes II, III, and IV were deposited by these solutions. The bulk of the evidence in favor of a hydrothermal origin for the minerals of these classes lies in their relationship with the magmatic minerals. Undoubted actual replacement of microcline by cleavelandite, an important product of the first hydrothermal stage, is described under the latter mineral. Other minerals of Classes II, III, and IV definitely replace minerals of the main pegmatite. In a similar way the later hydrothermal minerals replace the earlier. At Greenwood extensive silicification during the last stages of mineralization resulted in many quartz pseudomorphs. The ability to divide the minerals, due to their consistent associations, into definite classes is regarded as evidence of aqueous deposition. Direct igneous crystallization produces a sequence of mineralization, but the products are the result of one continuous phase. Deposition was probably fairly continuous in the central Maine pegmatites, but changes in the character of the ascending solutions produced rather abrupt differences in the resulting minerals. Further evidence of a hydrothermal origin is shown by the presence of many minerals often found in veins. The high temperature vein minerals, tourmaline, albite, topaz, and cassiterite, are found in Class II. A low temperature vein mineral, rhodochrosite, is found in Class III. Likewise, in this and the next class, several hydrous minerals appear, signifying low temperatures and abundant water. Lastly, the fact that these minerals are limited in extent to a relatively restricted zone in the pegmatite (intersected by the lower quarry floor) suggests that they were deposited there by solutions working along a local channelway. The cavities, or pockets, are of great interest. Some rather large ones have been uncovered in the central Maine pegmatites. The largest pocket at Buckfield extended from the open cut back into the ledge, with varying course, for over twenty-five feet. The pockets at Greenwood were smaller, although one measured seven feet wide and ten feet long, with a depth of four feet. Mount Mica has been famous for its pockets, the largest of which was 20 by 12 by 7 feet. The cavities vary from this extreme down to a diameter of only a few inches. When found they are partially filled with debris (pocket sand) which has scaled off from the walls and roof. Writers in the past have ascribed the origin of such cavities in pegmatites either to gas action or shrinkage during crystallization. In 1853 Delesse 3 said:It is then probable that the cavities are the result of the action of gases; their forms and their character indicate, moreover, that the gases were set free at the moment when the rock was sufficiently consolidated to preserve the cavities, but when it was still so plastic that the minerals could develop very freely. Brögger 4 states that drusy cavities result from contraction during crystallization. Miarolitic cavities are filled by both late magmatic crystallization and by minerals formed by special "agents mineralizateurs."A few years later Crosby and Fuller 5 expressed similar views:. . . . while a higher degree of local hydration. gives rise to the concentric crystalline shells and shrinkage cracks (pockets) with the highly perfect crystallizations of feldspar, quartz, fayalite, etc., characteristic of the typical lithophysae. On the subject of miarolitic cavities Bastin 6 says:Such cavities have been attributed by various writers to shrinkage of the pegmatite mass in crystallization. This may, in fact, play some part in their formation, but that they are not entirely the result of shrinkage, but, on the contrary, were filled or partly filled with some material which has since disappeared, is shown by the presence of perfectly developed crystals of quartz, tourmaline, and other minerals projecting inward from the walls of the cavities. Some filling must have been present from which such crystals derived the materials for their growth. It is probable, therefore, that immediately after the crystallization of the main body of pegmatite the miarolitic cavities were completely filled with a gaseous solution which may later have liquified and has since disappeared. Water carrying numerous other substances in solution probably formed the bulk of this cavity filling. The abundance of quartz crystals on the walls of these cavities indicates that silica was one of the most abundant of the dissolved substances. It is the writer's opinion that most of the pockets in the pegmatites of central Maine are secondary in nature, due to dissolving activity on the part of the ascending solutions, rather than primary gas or contraction cavities in the igneous magma. This conclusion is based upon the following lines of evidence: 1. Shape of pocket. The larger cavities are extremely irregular in shape. The dimensions of the largest one at Buckfield were 3 1/2 by 3 1/2 by 25 feet.. This tunnel-like opening follows a tortuous course and constantly varies in diameter. In describing a similar cavity at Greenwood Bastin 7 says: " . . . . numerous small lobes add irregularity to its form." The largest pocket at Mount Mica contained three connecting chambers." Surely there is nothing bubble-like or otherwise suggestive of gas cavities about such openings as these. In their irregularity they more nearly resemble limestone caves, which result from the dissolving activity of ground water.PLATE I
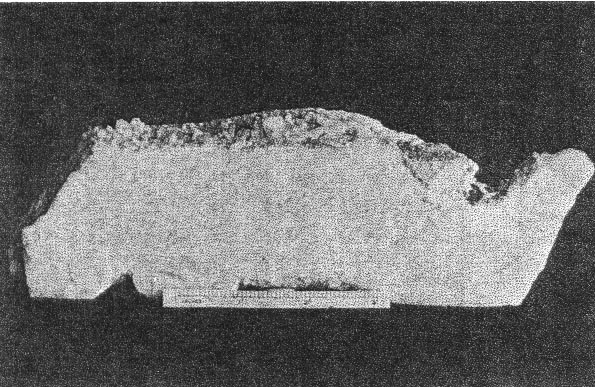
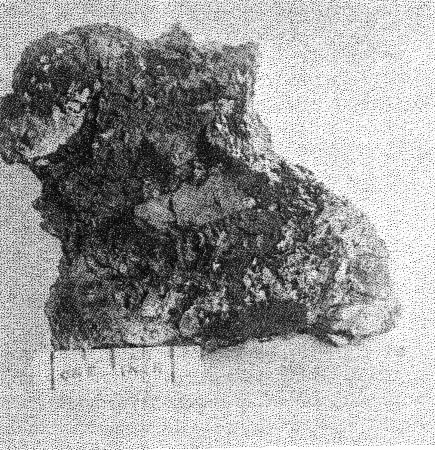
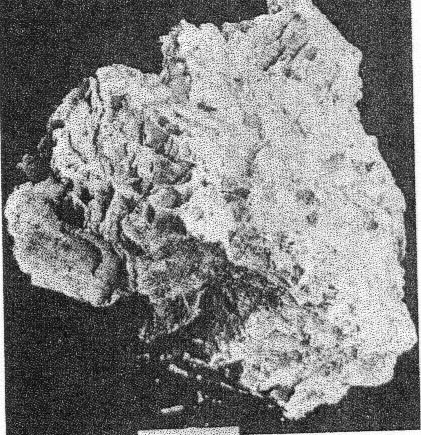
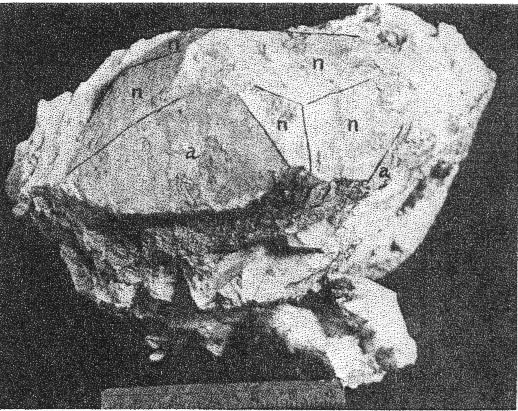
A. Microcline bordering a pocket. Note the extremely irregular profile caused by the dissolving activity of hydrothermal solutions. Quartz and cookeite (Class IV) coating the walls of the cavity. Buckfield.
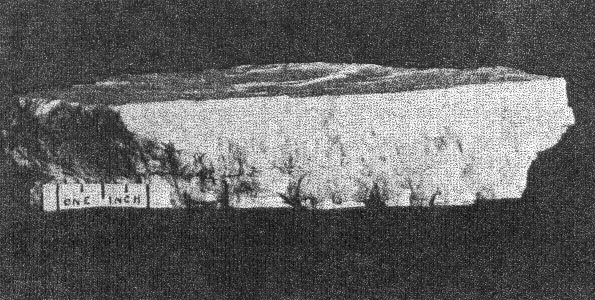
B. Cleavelandite in vein through microcline. The latter is a cleavage fragment of one individual of Class I. Buckfield.
2. The cross-cutting relationship of the pockets to the minerals of the main pegmatite. The cavities cut through the pegmatite as do dikes across sedimentary strata, with equal disregard for pre-existing structure. 3. Actual solution of microcline. Specimens of massive feldspar from Buckfield show unmistakable evidence of severe corrosion. Plate I, A pictures one such specimen. The microcline has been irregularly dissolved away, and the cavity walls are lined with hydrothermal minerals. Corrosion of a similar nature has been noted by Lacroix: 9Up to this period of formation of the rock it seems that the phenomena of crystallization were products of a continuous phase, with only the changes of chemical composition in the fluids circulating in the vein, these phenomena have been essentially constructive. But, there arrives a moment where a discontinuity intervenes; the minerals thus formed are no more found to be in equilibrium with the medium. They are subject then to corrosion and transformation. The great crystals of microcline have been profoundly corroded, attacked, in particular following their planes of cleavage. Cavities contain later albite, tourmaline, and lepidolite. 4. Anhedral forms of the magmatic quartz and microcline adjacent to the pockets. These minerals were prevented from assuming crystal boundaries in the main pegmatite by the lateness of their crystallization. But where they formed adjacent to a gas or shrinkage cavity one would expect crystal faces to be developed. Quartz crystals do line the pocket walls, but they are of a later generation. In the pockets under discussion microcline never has crystal boundaries. 5. Pseudomorphs. At Greenwood many quartz pseudomorphs have been found. The quartz was deposited about earlier minerals during the last hydrothermal stage. In most cases the minerals serving as host have been completely removed. This shows powerful dissolving activity toward the close of the hydrothermal period at least. 6. Invariable association of hydrothermal minerals with the large pockets. The secondary pocket zone is absolutely identical with the zone of hydrothermal minerals. This is to be expected if the same solutions were responsible for bath. The German writers, Laubmann and Steinmetz, 10 describe pegmatite cavities which they believe to be secondary:The distinct drusy development of these pegmatites as well as the magnificent crystal development in the druses, is secondary and brought about through the destruction of the rock. The possibility of cavities due to shrinkage or gas bubbles is not denied. It may be true that some or all of the secondary pockets in the central Maine pegmatites are merely much enlarged primary cavities. The pockets grew in size with each wave of hydrothermal activity and at the same time minerals were deposited, a different group for each period. Three such groups appear at Buckfield. The last activity was that of the surface waters forming the supergene minerals of Class V. CHEMICAL CHARACTER The chemical character of the pegmatite varies with its different phases. In the earliest phase the mass composition of the pegmatite magma was the controlling factor. This magma was very similar chemically to a granite magma, so the composition of the resulting pegmatite is close to that of a granite. Unusual amounts of lithium, manganese, and glucinum resulted in the formation of lepidolite, manganese apatite, and beryl among the magmatic minerals. With the completion of the magmatic crystallization and the subsequent hydrothermal activity the rarer elements of the magma are relatively more abundant while potash and alumina correspondingly decrease. Silica remains quantitatively important in Class II, almost disappears in Class III, and returns in large amounts in Class IV. In Class II we find the greatest variety of elements and minerals. The hydrothermal solutions carrying the residual liquor of the magma were loaded with all the elements of the original magma, except potassium and arsenic. Many elements appear in Class II which were lacking in Class I, and some of these, such as caesium, columbium, tantalum, and tin, do not appear in any later class. No new elements were introduced after the close of this earliest hydrothermal stage. From a chemical standpoint this class may be called the silica-sodium-rare element phase. The Class III minerals represent a lithium-manganese-phosphorus phase. These three elements were in the magma in unusual quantities and became increasingly important as the mineralization progressed from Class I to Class III. Manganese and phosphorus reached their climax here, but lithium did not. Hydrothermal activity closed with a lithium-silica phase, which produced the minerals of Class IV. Chemically combined water first appeared in Class II and increased in importance through Classes III and IV. Supergene water combined with hypogene elements to make many of the minerals of Class V. MINERALS OF CLASS I The minerals of this class make up the main body of the pegmatite and at most localities they stand alone, there being no evidence of later activity. At Buckfield many later minerals are found, but they represent a very small proportion of the total mass. he minerals of Class I crystallized from a magma. The analogy to a granite is apparently also followed in the order of formation. The first to complete their crystallization were the accessories garnet, beryl, tourmaline, and apatite. Then came feldspar and lastly, quartz. Physically the minerals were (and still are where removed from the zone of later activity) tightly intergrown and no interstitial open spaces were found which were left by this primary crystallization. In the relative size of grain the pegmatite does not differ widely from a granite. While the apatite, tourmaline, mica, and garnet crystals are several hundred times their customary size in a granite, so are the feldspar and quartz masses. A possible exception is beryl, which does not appear in granite. MICROCLINE Microcline is the most abundant mineral at Buckfield. The only other feldspar detected in Class I was albite, in perthitic intergrowth with microcline. Potash feldspar, although of extreme importance here, does not appear in any of the later classes. On the freshly cut quarry face microcline stands out prominently due to its white color and large cleavage faces. Some of the latter are two meters or more across and the grain varies from this dimension to about half a millimeter in the contact phase. Later dendrites of a black manganese oxide appear on many of the cleavage faces. Kaolinization of the microcline has been very slight, as seen under the microscope. The characteristic albite and pericline twinning is commonly absent, but the 15° extinction angle on the base distinguishes it from orthoclase. The feldspar appears to have completed its crystallization after all the other minerals save quartz. It encloses euhedral crystals of beryl, tourmaline, and garnet and is itself surrounded by massive quartz. Potash feldspar, usually microcline, is a leading mineral in the granite pegmatites of central Maine, and elsewhere. QUARTZ Quartz is next in abundance to microcline. It appears in large gray masses equal in dimension to those of feldspar. Nowhere are crystal boundaries developed. As in a granite the final crystallization of the quartz completed the solidification of the magma. Therefore, none but anhedral forms were possible, and those surround and enclose all of the other constituents. At the top of the hill a series of six-inch quartz veins with parallel strikes cuts the main pegmatite. APATITE Apatite appears scattered through the microcline in dark green, opaque, somewhat spherical anhedrons from a fraction of a millimeter up to fifteen millimeters in diameter and is clearly of late crystallization. Under the microscope it is almost colorless. Its indices of refraction are: ω = 1.642±.003, ε = 1.639±.003. Chemical analysis shows the mineral to be manganapatite, as it contains 8.69 per cent MnO. The complete analysis by Miss Vassar follows: P 2O5 41.43 per centCaO 47.33 Fe2O3 0.55 MnO 8.67 F 3.80 C1 ------ Al2O3 ------ H2O 0.06 101.84 F=O 1.60 100.24 percent Analyses are given in Dana's System of Mineralogy for eight manganapatites, only one of which definitely contained more MnO than this. A dark green apatite from Branchville, Connecticut, had 10.59 per cent MnO. This particular apatite is less common in the other central Maine pegmatites. In his description of Mount Mica Bastin 11 says, "Apatite occurs in the solid pegmatite in irregular opaque green masses, some few of which weigh a couple of pounds." Apatite of this character was observed at Greenwood, where the mineral is also exceptionally abundant as the product of a later mineralization.BERYL Beryl is common in quartz and feldspar at the northwest corner of the large quarry, and appears in even greater abundance in the small open cut four hundred feet beyond. Here hardly a square yard of the pegmatite face may be found which does not contain a section of a beryl crystal. The crystals are strongly prismatic, up to eight centimeters in diameter and twenty centimeters long. Some odd shapes appear. One crystal abruptly doubles its diameter, without displacing the vertical axis. Several crystals exhibit a decided tapering along the prism zone. The color ranges from bluish green to green, usually somewhat mottled. All in this class are opaque or translucent. TOURMALINE Tourmaline of the first class is here as in most pegmatites black and opaque and appears in well-defined prismatic crystals scattered rather widely through the main mass of the pegmatite. Some of these reach ten or twelve centimeters in diameter and twenty centimeters or more in length. LEPIDOLITE In the main pegmatite lepidolite has all the appearance of muscovite. It occurs in silvery white books, usually five to eight centimeters in diameter and under one centimeter in thickness. Optically lepidolite is almost identical with muscovite, so the flame test was used in distinguishing these two micas. The white mica of the first class gave as sharp and as definite a lithium flame as did the pink lepidolites of the later classes. The flame was secured all about the periphery, in the center, and in every quadrant of the book. Also the plates were microscopically homogeneous. Nowhere was there any evidence of lepidolite-muscovite zoning or intergrowths as described in specimens from Haddam Neck.12 No muscovite could be found among the specimens from Buckfield.Lepidolite also was produced to a minor extent about the biotite gneiss xenoliths found near the contact. Reaction between the biotite of the xenolith and the enclosing lithia-rich magma resulted in a zone of lepidolite 6 millimeters wide between the two. The mica of Class I is usually muscovite as is the case at Greenwood and Mount Mica. Evidently an unusual concentration of lithium in the pegmatite magma existed at Buckfield. Lepidolite occurs in the normal pegmatites in Madagascar. 13BIOTITE Biotite is an accidental constituent of the pegmatite, being introduced by the inclusion of fragments of biotite gneiss country rock. Working over by the magma produced a chemical and physical change in the biotite. The flakes are larger than they were in the gneiss, and lithium has been incorporated, due to its great abundance in the magma. The biotite is black with a most peculiar intergrowth of plates. These are all arranged parallel to one of two directions in the xenoliths. As the changes in direction are minutely spaced the result is two perfect cleavages, at right angles in one xenolith, and at approximately 120° in another. GARNET A brownish red garnet is a very minor accessory in the main pegmatite. Judging from its index of refraction, color, and associations it is very likely spessartite which is very common in the pegmatites of Maine. ARSENOPYRITE Only two specimens containing arsenopyrite were found. In one it occurred in parallel bands 1 to 5 millimeters thick. Between the bands were even narrower zones of brown lepidolite. The latter was in plates about 5 millimeters across which were arranged parallel to the banding. The whole is tightly enclosed in massive microcline and quartz. In the second specimen arsenopyrite appears in crystals and small masses in the finer grained pegmatite adjacent to the contact. Associated minerals are microcline and lithium biotite. Either arsenopyrite or löllingite the sulphur free iron arsenide, occur in most of the central Maine pegmatites. The former was present sparingly at Greenwood. Both were found at Mount Mica. AMBLYGONITE Amblygonite is very rare in the main pegmatite, appearing in but one hand specimen studied. Here it is a small cream colored fragment intimately associated with manganese apatite, tourmaline, and white lepidolite. Amblygonite has been reported from Mount Apatite and Mount Mica, from the latter locality in some abundance. MINERALS OF CLASS II Soon after the close of the magmatic period hydrothermal activity set in and the minerals of Class II were formed. The more unusual elements of the magma now come to the fore. Beside silica the solutions of the first stage carried unusual amounts of sodium, lithium, and the rarest elements. Many of these last were soon exhausted so do not appear among the constituents of the minerals in the later classes. The temperature at which the minerals of Class II were deposited was considerably below that in effect during the magmatic period. The presence of low temperature quartz places it below 575°C. But the temperatures were still high in terms of hydrothermal mineralization. This is shown by the presence of such typical high temperature vein minerals as tourmaline, albite, topaz, and cassiterite. The upward moving solutions dissolved away the minerals of the main pegmatite, producing pockets. In the pockets many minerals were deposited, some as drusy linings, others entering into and replacing the walls, while still others may have been formed without attachment, in the free liquid filling the pocket. The minerals of Class II may be divided upon a basis of quantitative importance into three major and ten minor minerals. The three major ones are quartz, lepidolite, and cleavelandite. Quartz was the first to form, cleavelandite the last. These are the characteristic pocket minerals of granite pegmatites. The minor minerals were all formed before cleavelandite, and some even before quartz. In most cases their exact position is unknown because of the scantiness of their occurrence. QUARTZ Quartz crystals of hydrothermal origin appear in a multitude of sizes and shapes and vary from colorless to milky or smoky. Euhedral crystals of the low temperature type are the rule. These may be perfect crystals, with equally developed prism and rhombohedron faces, or they may be flattened and distorted, usually parallel to a prism face. Many of the crystals are under 2 centimeters in length while a few attain lengths of over 2 meters. The majority fall between these two extremes. Crystal groups were common at Buckfield. One of these, measuring about 40 centimeters across, consisted of three quartz crystals whose prisms completely merged, giving a massive base to the specimen. Only the smaller crystals are colorless. The large ones from this locality are almost always milky. Smoky crystals are exceptional. The Class II quartz crystals line the pocket walls in great abundance. They were seen on the walls of the big pocket and groups of large crystals were common in the region of slumped cavities intersected by the lower quarry floor. Quartz is the most spectacular of the pocket minerals at Buckfield, due to the abundance and size of its crystals, the variety of its forms, and its drusy occurrence. On the basis of form alone may the hydrothermal quartz be distinguished from the magmatic variety. The latter is never euhedral, while Class II quartz is rarely otherwise. Associated minerals are cleavelandite and lepidolite. Quartz formed before these minerals, for the latter are often found, clinging to the sides or partially rooted into the quartz crystals. LEPIDOLITE The lepidolite of Class II has the characteristic lilac color. It appears in books usually under a centimeter in diameter and up to three centimeters thick. Hardly a specimen containing minerals of Class II fails to show lepidolite. Furthermore, it was one of the most stable of the pocket minerals as in many specimens the only remnants of the first hydrothermal stage are a few flakes or small books of lepidolite, the later solutions having dissolved or replaced the associated minerals. Pocket lepidolite may readily be distinguished from the earlier variety. It is generally lilac rather than white in color. The books are small and in most instances were formed in open spaces. The much larger books of Class I lepidolite are tightly enclosed and often subhedral. All the pocket-bearing pegmatites in central Maine contain Class II lepidolite. It was probably more abundant at Mount Mica than at any of the other localities. The constant association of lepidolite with pocket tourmaline is well known to the gem hunter. CLEAVELANDITE The platy variety of albite, cleavelandite, is one of the commonest pocket minerals. It is not found outside of Class II and is therefore an unfailing index of the first hydrothermal period. The Buckfield cleavelandite is found in single crystals, sheaves of plates, and fan-like aggregates. It is white and usually translucent although one group contained several parallel transparent crystals. The plates vary in thickness from very minute up to 8 millimeters but most are considerably under 3 millimeters.
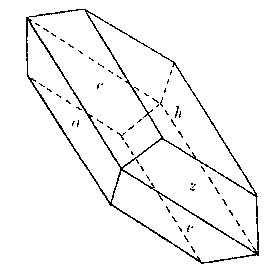
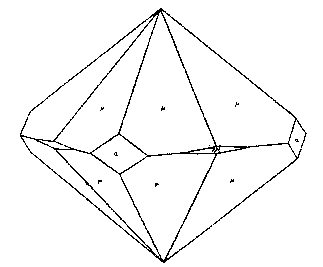
The crystals of cleavelandite are invariably twinned on the albite law. One group showed crystals of sufficient
perfection to be measurable and the following forms, some of them quite rare, were identified: b(010), c(001),
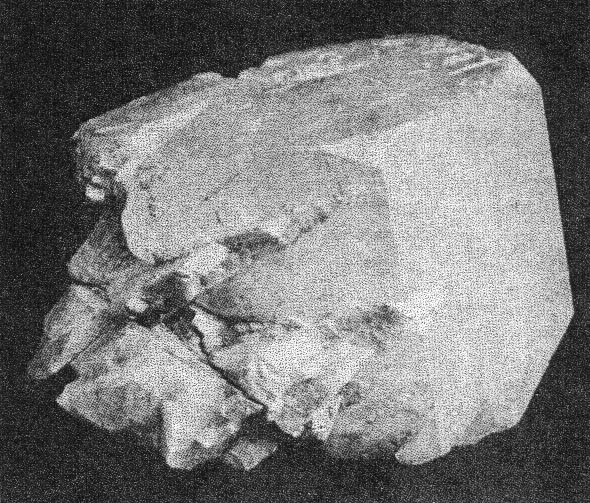
m(110), M(1 Cleavelandite usually replaces earlier minerals, although it may also fill open spaces. One of the easily replaced minerals was microcline. Several specimens were found with veins of cleavelandite through the microcline. The depositing solutions had worked into cleavage cracks in the magmatic feldspar and the cleavelandite replaced outward from this plane of weakness. One of these specimens is shown in Plate I B. Cleavelandite replaces quartz also. Many of the pocket crystals of quartz have been entered by blades of this mineral. In fact aggregates of cleavelandite largely filling open spaces usually anchored themselves in this manner. All of the pocket minerals are closely associated with cleavelandite. Quartz and lepidolite are the commonest of these. The rarer minerals, tourmaline, columbite, spodumene, apatite, etc., are found either embedded in or adjacent to cleavelandite. The latter appears to have been the last of the Class II minerals to form.Platy albite was equally important among the pocket minerals elsewhere. It was found in fair amounts at Greenwood, Mount Mica, and Auburn. In every case cleavelandite was a product of early hydrothermal activity. This mineral is a most important criterion of the proximity of pockets. BERYL The beryl of Class II rarely exhibits crystal boundaries. Its solubility in the later solutions has usually resulted in a severe etching, which has destroyed the outlines of the original crystal. These etched forms are known as "pocket" beryls, because of their characteristic occurrence in the vugs of pegmatites. One white transparent crystal was evidently partly buried in the wall and partly emergent into the pocket, for one-half the crystal is highly etched, while the other half is unharmed, with crystal boundaries intact. Another larger crystal shown in Plate II A survived the attack of the later solutions even better. This crystal is about 10 centimeters long and 15 centimeters in diameter. The first order prism and basal pinacoid are dominant, the second order pyramid subordinate. The faces of the last form are etched. The crystal is pale aquamarine in color and unusually transparent. The pocket beryls vary in color from pale green or colorless to white and pink, and are always far more transparent than the green or blue beryl of Class I. Besides these differences the beryls of Class II vary from the magmatic crystals in specific gravity and in indices of refraction, as shown below:
Class I Class II The higher specific gravity and indices of refraction are probably due to an unusual amount of alkalis, especially caesium. The presence of caesium in the ascending solutions is shown by the occurrence in this class of pollucite, a caesium silicate. Pocket beryl is known throughout central Maine as caesium beryl, due to the presence of caesium in beryl of this type from Hebron. PLATE II
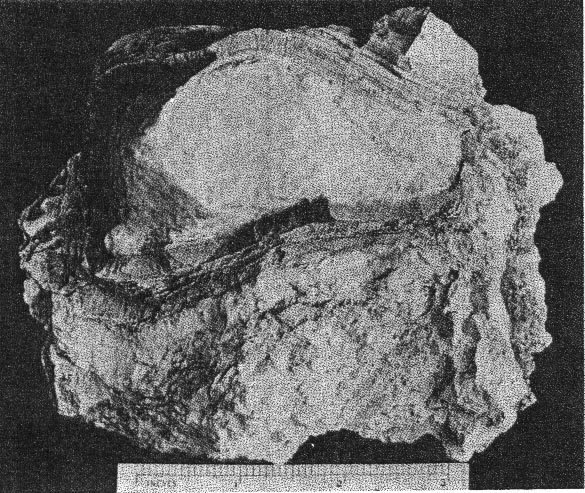
A. Pocket beryl crystal from Buckfield. Cleavelandite at lower left. About half natural size.
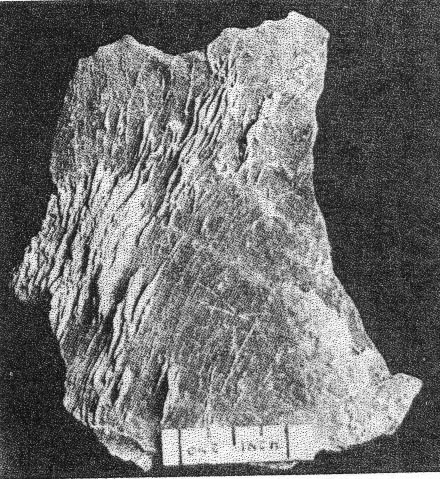
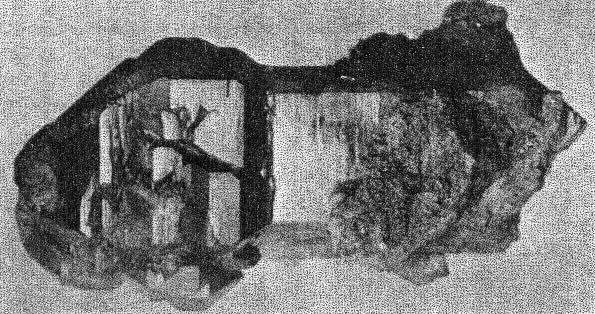
B. Eosphorite crystals in a base of rhodochrosite and fairfieldite. Supergene rhodochrosite coats the latter minerals. Buckfield. Class II etched beryls are common at Greenwood and some good crystals have been found in the pockets in the pegmatite on Mount Mica. TOURMALINE One of the first pockets opened at Buckfield contained two fine gem crystals of tourmaline, five centimeters long. Many small clear green unattached tourmaline crystals were found later. A few very small crystals also appear among the lepidolite, cleavelandite, and quartz specimens. In one of these a needle-like tourmaline is included within a quartz crystal, making the tourmaline definitely earlier in age. Study of the pocket tourmaline crystals from Buckfield and elsewhere shows that some worked into the walls, replacing earlier minerals, but many more grew out from the walls, one end attached, the other free. Subsequent movements, perhaps sometimes those due to the blasting of the quarrymen, often shook these crystals free, and they fell to the bottom of the pocket. Here they are found loose, mixed with the other debris of the pocket. Sometimes very thin needle-like and doubly terminated tourmalines are found in this pocket sand. Such an occurrence was observed by Dr. Palache 14 at Haddam Neck, Connecticut. These crystals were probably never attached, but were formed in the solutions which filled the pocket during the first hydrothermal period, and were left free in the bottom of the pocket with the passing on of the solutions.The tourmaline of this class differs strikingly from that belonging to the magmatic period. It is green and transparent, whereas the tourmaline of Class I was black and opaque. The gem tourmaline is a result of early hydrothermal activity rather than the product of magmatic crystallization and contains more lithia and less iron than the black tourmaline of Class I. An increase in alkalis and a corresponding decrease in iron apparently cause increased transparency. Class II tourmaline crystals were common at Greenwood and in some of the Auburn quarries, but it was at Mount Mica that the mineral was most abundant. The large clear green and pink tourmalines from this locality have made it famous. SPODUMENE Most of the spodumene at Buckfield has been rather completely kaolinized. Some of these pseudomorphs are about eight centimeters long and half as wide. They are white and fibrous, exhibiting the exact structure (cleavage and parting) of the original mineral. A little lithium is present, undoubtedly due to residual spodumene. Spodumene pseudomorphs occur closely associated with cleavelandite and Class II lepidolite. The original spodumene formed after the lepidolite, sometimes completely enclosing it. Veins of lepidolite, belonging to a later period of mineralization, cut through several of the kaolinized spodumene specimens. Others have been irregularly stained along the planes of separation by a black manganese oxide. Much larger and fresher crystals of spodumene were found at Greenwood. Bastin 15 says that spodumene is "common in many of the coarser pegmatites, especially those that carry gem tourmalines, lepidolite, and other lithia minerals."APATITE Apatite is comparatively rare among the minerals of Class II. It appears in small green translucent crystals, prismatic in habit. Associated minerals are cleavelandite, lepidolite, and columbite. The apatite crystals are well developed and were formed previously to the enclosing cleavelandite. This apatite is rather different from the earlier magmatic apatite. Although both are green, the later apatite exhibits definite crystal boundaries and is translucent whereas the apatite of Class I is always anhedral, cloudy, and usually occurs in much larger masses. Pocket apatite of this generation is not uncommon in the granite pegmatites of central Maine, but the drusy apatite appearing in great abundance at Mount Apatite and Greenwood belongs to a later period of hydrothermal activity. HERDERITE Several pale green crystals of herderite were found embedded in cleavelandite. Others either clung to the sides of lepidolite books, or partially enclosed them; hence the mineral must have been deposited after the lepidolite but before the cleavelandite. The largest crystal is 4 centimeters long, 1.2 centimeters wide, and a centimeter thick, but most are about a centimeter long. Many of these smaller ones are twinned.Herderite was relatively insoluble during the later hydrothermal activity, for one specimen consists of a small vug containing herderite and lepidolite crystals completely surrounded by quartz and cookeite of Class IV. This is the remnant of a pocket and its minerals established during the first hydrothermal period. Herderite is a rather rare mineral. In this country it is found at Stoneham, Hebron, Greenwood, and Auburn, Maine. COLUMBITE Black, platy and prismatic columbite crystals up to 5 centimeters in length were occasionally found, usually embedded in cleavelandite. The latter is distinctly later, as it pierces the columbite in salients and veins. A bright purple oxidation product coats many of the crystals. Compared with its associates columbite was very insoluble in the later solutions. Therefore it is often found in residual remnants enclosed in minerals of a much later generation. Small amounts of columbite were noted at Greenwood, Mount Mica, and Auburn. It is widely distributed in New England pegmatites. MANGANOTANTALITE A few small masses of manganotantalite appear, encroached upon and surrounded by quartz, cookeite, and apatite of Class IV. They are all under one centimeter in diameter, and in most instances have lost their crystal boundaries, due to the extensive attack of the solutions depositing the later minerals. With the exception of a few flakes of lepidolite the associated Class II minerals succumbed to this attack, and the manganotantalite and lepidolite are the only remnants of the earlier mineralization. The Buckfield manganotantalite is dark brown and slightly translucent. Its gravity is 7.29. Under the microscope the mineral appears yellow in color with no pleochroism discernable. It is biaxial positive, with n approximately 2.2. Columbite has higher indices of refraction, lower specific gravity, and is more opaque. The common ferrotantalite is pleochroic and darker colored. Manganotantalite from Mount Apatite has been described by Schaller.16CASSITERITE Cassiterite, a fairly common accessory mineral in granite pegmatites, is represented in the Buckfield collection by an isolated black pyramidal crystal about two centimeters long. No other minerals were attached to it to give a clue to its place in the genetic classification. It was however placed here in Class II because of its associations elsewhere. At Mt. Mica cassiterite was not uncommon in cleavelandite and in the pocket sand. It was also so found at Greenwood and in most New England pegmatites. POLLUCITE A few small fragments of this rare mineral, a hydrous caesium aluminum silicate, were found at Buckfield. It is white and glassy and lacks any very striking physical characteristics that are distinctive. The only associated mineral is lepidolite, in vein form and obviously much later. While pollucite has been found in small amounts in several of the Maine pegmatites the only abundant occurrence of it is in Dudley's ledge, also in the town of Buckfield and about two and a half miles south of Bennett's quarry. TOPAZ The single crystal of topaz found at Buckfield is a prismatic individual about 5 centimeters long and four in diameter. It shows faces of basal pinacoid and brachydomes which are coated with fine scales of purple lepidolite belonging to Class IV. The common occurrence of topaz with cassiterite and with cleavelandite as at Stoneham is the reason why it is here placed in Class II. TWO UNKNOWN PHOSPHATES Two unidentified minerals were found mixed with quartz and lepidolite in one specimen. Difficulties encountered in their separation prevent a complete description at this time. The two are intimately associated and megascopically the aggregate looks homogeneous. It is white to colorless, quite vitreous and has a hardness of about 5. Under the microscope the more abundant of the two minerals is found to have a good cleavage parallel to the base. Many of the particles contain minute rectangular inclusions with much higher indices. This mineral has the following optical properties: Uniaxial +, ω = 1.591, ε = 1.606. An incomplete chemical analysis was made by Miss Vassar on a sample of this mineral which contained a small amount of lepidolite. The results are given below:
The alkalies may account for the missing nine per cent and if this is true the mineral is a hydrous phosphate of aluminum and the alkalies. The second mineral is biaxial negative, but with 2V small. The indices are: α = 1.640, β = 1.656, γ = 1.661. Definite microchemical tests proved the mineral to be a phosphate. The optical data on neither of these minerals agree with those given for any phosphates listed in Larsen's tables. Final determination must await further work in separation and analysis. MINERALS OF CLASS III A second wave of hydrothermal activity produced the minerals of Class III. This mineralization represents a lithium-manganese-phosphorus phase in the ascending magmatic waters. At Buckfield seven minerals were formed, the majority of which are very rare. The phase itself is unusual in pegmatite formation and it is found elsewhere in this country developed to any degree only at Branchville, Connecticut.17 Pegmatites containing phosphate minerals in which iron predominates over manganese are found in France,18 and in Rabenstein, Bavaria.19The minerals of Class III were deposited in a definite sequence, with but little chemical change from one mineral to the next. A slight difference in the character of the ascending solutions would cause an earlier mineral to become unstable and a new one to be deposited. The later mineral probably grew at the expense of earlier minerals, incorporating in itself some of the material dissolved from them. A tabular view of the chemical and mineralogical changes occurring during this period is given below. The minerals are listed in the order of their formation and the lower half of the table includes similar minerals of Class IV and the supergene minerals of Class V in order to show the time relations of the whole phosphate group. |
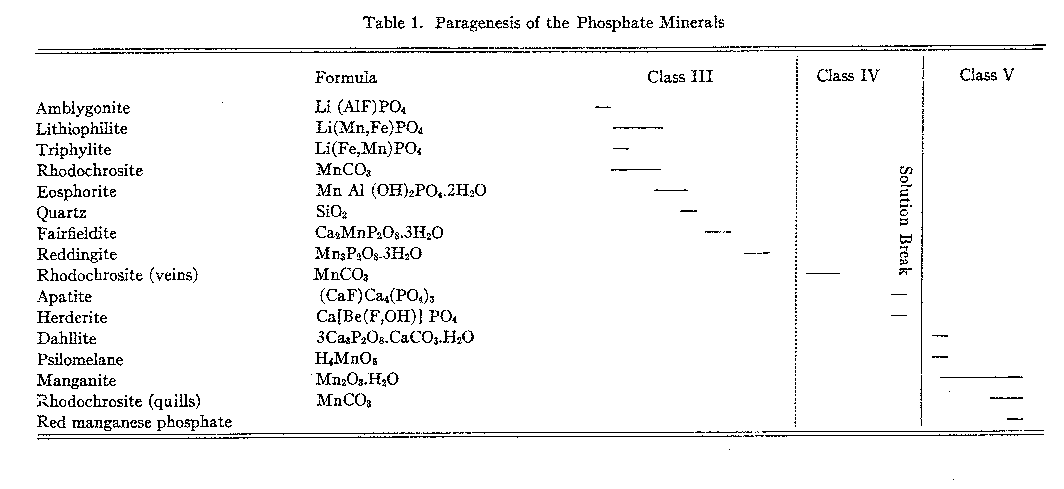
Lithium disappears as a mineral constituent after the formation of lithiophilite and triphylite and does not reappear until Class IV. Manganese is of utmost importance in all but the first mineral. Iron is very subordinate, as the chemical analyses will show. One carbonate, rhodochrosite, interrupts the sequence of phosphates. Most, if not all, of the Class III minerals were deposited under low temperature conditions. Rhodochrosite is typical of shallow veins as are the hydrous minerals. The method of mineral formation varied during the period as in the first half replacement was the general rule while later open space filling became dominant, although the minerals were often replacive in character also. Other evidence than temperature differences place the Class III minerals later in age than those belonging in Class II. In one specimen actual superimposition of minerals was observed. Here lithiophilite, a leading mineral of Class III, is definitely replacing spodumene, one of the minerals typical of Class II. AMBLYGONITE A grayish white rim surrounds the pocket minerals of Class III. The border is about 4 centimeters wide, and usually consists of material microscopic in dimension. The rim is always bounded on the outside by massive microcline of the main pegmatite. It is evidently caused by a replacement of the microcline. Optical study and chemical tests proved the replacing mineral to be amblygonite. Residual fragments of microcline surrounded on three sides by amblygonite may be seen along the outer margin of this zone in the thin section. Cleavage cracks in the feldspar are filled with amblygonite. The main Class III mineralization was preceded by an amblygonite stage, during which this mineral advanced outward into the main pegmatite, replacing microcline as it did so. In a few specimens replacement has been complete, and the amblygonite is coarse enough to show cleavage and yield good blowpipe reactions. Optical differences serve to distinguish amblygonite of Class III from that of Class I, as shown below:20
Class
I
Class III LITHIOPHILITE Lithiophilite is orthorhombic with perfect basal and good side pinacoidal cleavage. At Buckfield it occurs as it does at Branchville, in anhedrons, single individuals sometimes measuring as much as ten centimeters across. It is pale salmon pink in color and for the most part very fresh. Under the microscope the pleochroism usually assigned to this mineral is lacking and it is colorless in all directions. The indices of lithiophilite from both Buckfield and Branchville, Connecticut, the type locality, were measured. They are given below:
Buckfield (FeO=2.94%) Branchville (FeO=12.57%) The indices increase with higher percentages of iron. In composition lithiophilite is a phosphate of manganese and lithium with but little iron. It is isomorphous with triphylite which contains much iron and little manganese and is blue in color. Chemical analyses were made of the lithiophilite specimens whose indices were measured. The specimen from Branchville was collected in 1924 from the new openings. Analyses of lithiophilite by Miss Vassar.
The least FeO previously reported was 3.99 per cent, on a different specimen from Branchville.21 The Buckfield material with but 2.96 per cent FeO is the nearest of any lithiophilite so far analyzed to the theoretical end point of the triphylite-lithiophilite series. At Buckfield lithiophilite occurs frozen to the pocket walls, which generally consist of massive quartz. A change in the character of the solutions following the deposition of lithiophilite made this mineral unstable, and it was extensively attacked and replaced by later minerals. Beside its well known occurrence at Branchville lithiophilite has been reported from Tubbs Farm, Norway, Maine; Faires Mine, 22 Cleveland County, North Carolina; and Pala, California. A specimen from the latter locality in the Harvard collection shows lithiophilite with sicklerite, a more hydrous lithium-iron-manganese phosphate.TRIPHYLITE Triphylite is represented in the Buckfield collection by a single isolated fragment measuring five by five by two and a half centimeters, blue in color. Owing to its chemical similarity with lithiophilite it is assumed that triphylite belongs in Class III as well. As no other minerals were present in the specimen its paragenesis could not be determined further. Probably a very temporary increase in the amount of iron in the ascending solutions was responsible for the formation of a small amount of triphylite at Buckfield. Triphylite has a wider geographical range than lithiophilite. It has been found at Mount Mica, Stoneham, and Peru, Maine; Grafton, New Hampshire; Norwich, Massachusetts; Rabenstein, Bavaria; and Keityo, Finland. At several of these localities it is associated with spodumene.RHODOCHROSITE
The rhodochrosite of Class III has the pink color typical of the mineral. It appears occasionally in distinct, small
crystals showing the forms M (4041); f (02 A chemical analysis by Miss Vassar of the Buckfield rhodochrosite belonging to this generation follows:
FeO 2.97 per cent MnO 58.79 100.19 per cent This is the only appearance of a primary carbonate. For a short period the proportions of CO 2 to P2O5 in the ascending solutions must have been such that the former displaced the latter as the negative radical.No specimens could be found containing rhodochrosite and lithiophilite adjoining, so the relative age of these two minerals is in doubt. Like lithiophilite, rhodochrosite has been partially dissolved by subsequent solutions, and replaced and coated by later minerals. Rhodochrosite is rare among the pegmatites of central Maine. A small quantity was found at the Towne quarry, Auburn, and at the Berry quarry in Poland. At Branchville this mineral was common with the manganese phosphates found there. EOSPHORITE The long yellow prismatic crystals of eosphorite make it the most striking mineral in Class III. A picture of a group of such crystals is shown on Plate B. The mineral is orthorhombic with the direction of elongation taken as the vertical axis. The faces of the prism zone are striated vertically. Forms and habit of the crystals are identical with those of the type material from Branchville, Connecticut. The refractive indices of this eosphorite are as follows: α = 1.629, β = 1.650, γ = 1.658. These are slightly below those given for a higher iron variety from Branchville. 23The ideal eosphorite is a hydrous phosphate of manganese and aluminum. It is isomorphous with childrenite, which contains iron instead of manganese. A chemical analysis of the Buckfield eosphorite by Miss Vassar is given below: SiO 2 0.90 per centAl2O3 22.37 MnO 29.94 FeO 1.38 H2O± 15.34 F trace P2O5 29.89 99.82 per cent Buckfield eosphorite follows lithiophilite in closely approaching the theoretical end point of the series. Its content of FeO, 1.38 per cent, is much lower than that in any of the childrenite-eosphorite analyses published in Dana or Doelter. The Branchville eosphorite contained 6.62 per cent FeO. With the introduction of eosphorite chemically combined water becomes important. This and the succeeding minerals in Class III contain fair amounts of water. Determination of the relative age of eosphorite is complicated by two features of the mineral. One is its strong crystallization power which tends to mask its true relationship with associated minerals. The other is its unusually high resistance to attacking solutions, which have in some instances removed the neighboring minerals to such an extent that eosphorite will rest upon and appear later than younger minerals. Although on the whole eosphorite deposition followed the formation of rhodochrosite there was a slight overlap of the two. This is shown on one specimen where a large crystal of eosphorite started to grow out from a rhodochrosite mass only to have the latter add a little to its size, burying the stump end of the eosphorite crystal a fraction of an inch. This and other examples of eosphorite crystals lying on and growing out from rhodochrosite masses prove the slightly later age of eosphorite.Connecticut, and Poland, 24 and Hebron, Maine. Specimens from Hebron have been called childrenite, but after an optical study Larsen stated that the mineral was probably eosphorite.25 Childrenite has been found at Mount Mica and at four English localities, one in Cornwall and three in Devonshire.Eosphorite from Mount Mica has not heretofore been described. Specimens in the Harvard laboratories contain this mineral in small radiating needles coated by dahllite. It is embedded in massive siderite. Under the microscope the Mount Mica eosphorite is pale yellow green, whereas the Buckfield material is colorless. The color and slightly higher indices show that the Mount Mica eosphorite is higher in iron than that from Buckfield, but it is not childrenite because the axial angle for violet is greater than that for red. FAIRFIELDITE Straw colored plates here and there among the Class III minerals were found to consist of small crystals of fairfieldite. These are triclinic, have one perfect cleavage, and in thin plates are absolutely colorless and transparent. Crystals of simple form were seen but proved to be too dull for measurement. The largest crystal, shown in Plate III, A, and nearly 3 centimeters long, is entirely coated with dahllite. Fairfieldite from the zone of oxidation is white and opaque and often coated with manganite. The nature of the alteration may be seen under the microscope. A white opaque mineral which may be kaolin has commenced to attack the fairfieldite, working first along the cleavage cracks and from them laterally into the mineral. Sometimes a yellowish to olive green alteration product is present but chemical and optical tests on this mineral were hopelessly complicated by the unfailing presence of residual fairfieldite. The refractive indices on the Buckfield fairfieldite were determined as follows: α = 1.633, β = 1.641, γ = 1.652. PLATE III
A. Dahllite coating various minerals of Class III. The large coated crystal in the center is fairfieldite. The stalactite looking forms are crystals of eosphorite coated with dahllite. Buckfield.
B. A crust of lepidolite. Formerly a vein, this fragment has been isolated by the solution of the host. The crust is under 3 millimeters in thickness. Note the exceedingly thin secondary veins projecting from the much larger main vein. Buckfield.Fairfieldite is a hydrous phosphate of calcium and manganese, with iron generally replacing a little of the manganese. The iron in the Buckfield material was very low. An analysis by Miss Vassar follows: SiO 2 1.07 per centFeO 1.00 CaO 29.77 H2O± 9.94 P2O5 37.79 MnO 19.68 99.25 per cent The lowest percentage of iron in previously analyzed fairfieldite was 3.42 per cent, in a specimen from Branchville. This is the only appearance in Class III of calcium in more than a trace. In fact, fairfieldite is one of the few minerals in the entire suite to contain this element. The calcium that was present in the ascending solutions during the other periods was usually taken care of by apatite, which does not appear in Class III. Fairfieldite was deposited after eosphorite. In some instances eosphorite crystals cut through fairfieldite and appear later in age, but a close examination shows that the cleavage directions in the fairfieldite on opposite sides of the eosphorite are never parallel. The latter does not divide single individuals, but merely lies between separate crystals, of later age. The type locality for fairfieldite is Branchville, Connecticut. Sandberger described an occurrence of this mineral, first under the name of leucomanganit, at Rabenstein, Bavaria. 26 Oberpfalz, Germany, is another reported locality.REDDINGITE Reddingite, a rose pink to reddish brown mineral, crystallizes in octohedroids of the orthorhombic system. It is usually granular, and ranks with rhodochrosite as one of the commonest minerals in Class III. It may be distinguished from that mineral by its lack of cleavage. The Buckfield reddingite is pleochroic. This serves to distinguish it in the thin section from lithiaphilite. It also has a much higher birefringence. The indices follow:
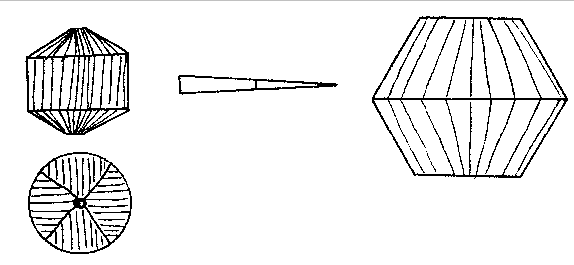
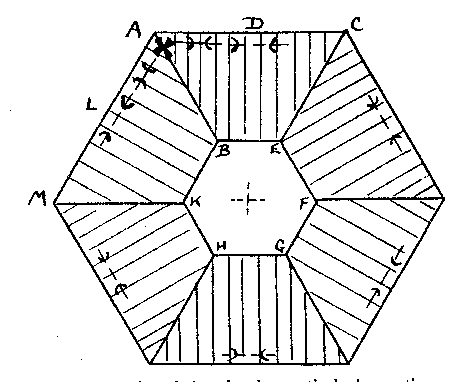
α = 1.643. X=colorless. The indices of the Branchville reddingite average .008 higher. A greater percentage of iron in the latter material probably accounts for this difference. Reddingite is a hydrous manganese phosphate, 3 MnO, P 2O5, 3H2O, and as in fairfieldite a small amount of iron replaces the manganese. An analysis of the Buckfield reddingite by Miss Vassar is given below:Insol. (SiO 2) 0.30 per centFeO 2.19 MgO 0.35 CaO 0.73 H2O± 13.14 P2O5 34.54 MnO 48.15 99.40 per cent The Branchville reddingite contained 4.70 per cent FeO. Reddingite is definitely later than all the other minerals in Class III. Previous to its deposition the ascending waters became active solvents and removed in part the preceding minerals (except the extremely insoluble eosphorite) forming cavities which later became lined with reddingite. The mineral also replaces, as veinlets in the lithiophilite are very common. Heretofore reddingite has been known only at Branchville, and there in very small quantities. The abundance at Buckfield of this rare mineral is remarkable. The very rare crystals found with idiomorphic form are identical in habit with the type crystals from Branchville. MINERALS OF CLASS IV Class IV is divided into two periods of mineralization. In the earlier period veins of lepidolite were formed in the zone containing Class II minerals, while veins of rhodochrosite were formed in the zone of Class III minerals. Both are clearly later than the same species with which they are thus associated. The confinement of these vein minerals to zones containing earlier deposits of the same minerals suggests that they did not result from an influx of new material at this stage, but were formed by solution and redeposition of material already in place. The waters bringing this about were probably hypogene as we know from the presence of later minerals that hydrothermal activity had not yet ceased. The later and main period of Class IV mineralization closed the hydrothermal activity at Buckfield. It represents a lithiasilica phase in the ascending solutions. Cookeite, quartz, apatite and herderite were deposited in the order named. The first two appear in great abundance. The latter are of very minor importance. These four minerals represent a well defined group, and were deposited so closely together that they overlapped. The vein minerals were deposited largely in fissures through earlier minerals. Cookeite and quartz also filled open spaces created by the solution of pre-existing minerals. The filling was far from perfect, as great porosity exists in the later Class IV specimens and in some the open spaces may be measured in inches. Volume by volume replacement was less common and was mostly confined to the alteration of vein lepidolite into cookeite. The Class IV minerals are quite evidently late in age, as shown by the cross cutting relationship of the lepidolite and rhodochrosite veins and the superimposition of quartz and cookeite on the minerals of the earlier classes. In some specimens such typical Class II minerals as cleavelandite, lepidolite, and milky quartz are coated by cookeite and quartz. In other cases removal of earlier minerals was far more complete. Often only a few bits of the difficultly soluble minerals are left in patches in quartz and cookeite as remnants of an earlier period. Lepidolite, columbite, manganotantalite, and kaolinized spodumene occur in this manner. In one specimen quartz and apatite are replacing rhodochrosite and amblygonite of Class III. A final phase of this sort in the deposition of pegmatite minerals is the rule rather than the exception in central Maine. At Greenwood it was an outstanding feature. LEPIDOLITE In Class IV lepidolite occurs exclusively in veins. This habit easily distinguishes it from the earlier hydrothermal and magmatic types. The veins are lilac in color, and cut minerals of Class II, especially spodumene. The separate individuals in the veins are very minute. They vary sufficiently in orientation so the vein has no well-defined cleavage. In the majority of cases the veins have been isolated by the complete removal of surrounding minerals. The result is an irregular sheet of massive lepidolite ranging from paper thin up to one centimeter in thickness. These are found buried in kaolin in the bottom of the pockets. Very often minor veins of much smaller dimension extended out from the large veins into cleavage and other cracks in the surrounding minerals. In that case a great host of excessively thin shelves jut out from the side of thicker slabs (Plate III, B). Their rough parallelism suggests that they were formerly veins extending into a highly cleavable or platy mineral, such as cleavelandite.One specimen contains as remnants of Class II mineralization a few quartz crystals and many small books of lepidolite. The latter are connected to each other by paper thin isolated veins of lepidolite. The plane of the vein is at right angles to the plane of the basal cleavage in the book lepidolite. Larger veins cut across the specimen in a few places. These likewise have a multitude of smaller offshoots. The secondary veins all have the same general strike, although often joining or diverging down the dip. No trace of the mineral or minerals serving as host for this deposition remains. RHODOCHROSITE Small veins of rhodochrosite cut through the minerals of Class III. These are obviously later than the massive rhodochrosite and phosphate minerals of that class. In many instances the earlier minerals have been removed by solution and dike-like forms are the result. These isolated veins are roughly parallel to each other except for an occasional vein which intersects the others at a low angle. Deposition of rhodochrosite continued during the period of solution of the neighboring minerals and as a result the projecting veins are lined with small pink rhodochrosite crystals, showing that ascending hot waters dissolved and redeposited rhodochrosite over a relatively long period. COOKEITE 27Cookeite is an abundant and rather conspicuous mineral at Buckfield. Large specimens were obtained coated with brownish aggregates of plates shown schematically in Fig. 2. The substance is so soft that the slightest touch broke the edges of these plates leaving a light yellow mark very much as the hand leaves a mark when it touches the bloom of a plum. The color also varies to pale green and pink. It may readily be distinguished from lepidolite by lower refractive indices and positive optical character. Under the microscope the plates of cookeite appear hexagonal as in Fig. 3. Some of the plates have a uniaxial center, e.g., BEFGHK, but others lack this central core. The uniaxial character is probably due to the aggregate effect of three plates at 60° to each other, the axial angle being largest midway in each segment. For example, in the segment ACEB the axial angle is largest at D with the plane of the optic axis parallel to the side AC; approaching A the angle gets smaller until on the line AB the axial angle is 0, then swings 60° and becomes parallel to AM, enlarging until it reaches the position L. The segments are striated perpendicular to their outer edges. The axial angle reaches a maximum of about 80 degrees.Some of the plates have narrow rims which are dark colored and may be due to an alteration of cookeite. One of these borders was large enough to note that the index was slightly less than the index of the cookeite.
Fig. 2. Cookeite. The indices of cookeite, determined by daylight, are: α = 1.576 ±.003; β = 1.579±.003 (seems to be constant in value); γ = 1.597 ±.005.The cookeite was separated by heavy solutions for analysis. The specific gravity of the clean sample was then determined by the pycnometer as 2.670; (according to Brush the sp. g. is 2.70, according to Penfield 2.675).
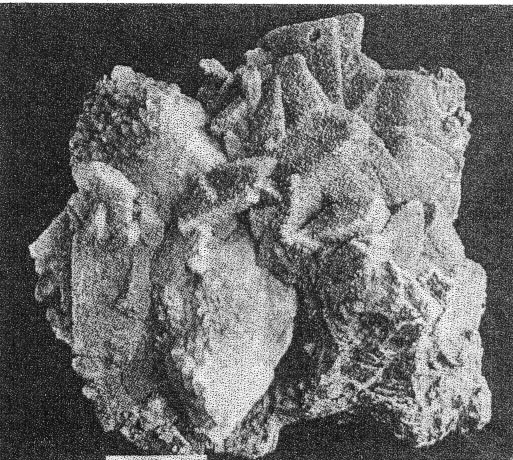
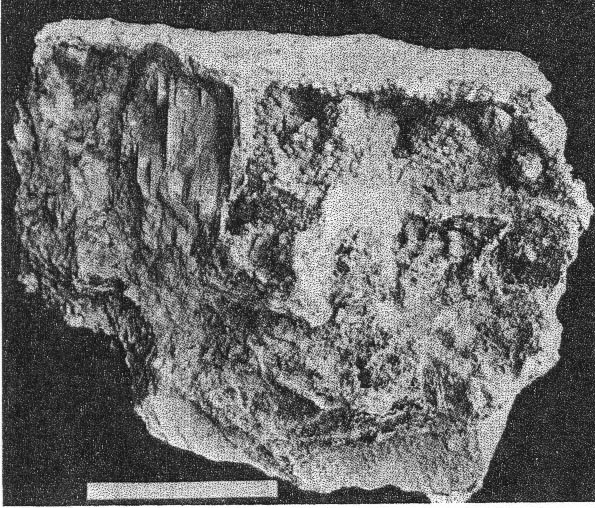
FIG. 3. Basal plate of cookeite showing optical observations and pseudohexagonal habit. Chemically cookeite is very similar to lepidolite. The chief differences are a greater percentage of water and correspondingly smaller amounts of fluorine and potash in cookeite. An analysis of Buckfield cookeite by Miss Vassar follows: SiO 2 34.81 per centAl2O3 45.90 Fe2O3 0.72 CaO trace Na2O } 0.52 K2O } H2O± 14.87 F 0.13 MnO trace Li2O 3.59 100.54 per cent The green cookeite often occurs lining solution cavities and cleavage faces in microcline. Later quartz crystals rest upon the cookeite. Plate I, A pictures a cavity coated in this manner. Solution and removal of microcline coated by quartz and cookeite may leave behind a mass of empty shells. Plates IV, A, and IV, B illustrate two stages in this process. Lepidolite, cleavelandite, and early hydrothermal quartz minerals of Class II are all coated locally with a later incrustation of cookeite. In one specimen cookeite had replaced spodumene, preserving the structure of that mineral. PLATE IV
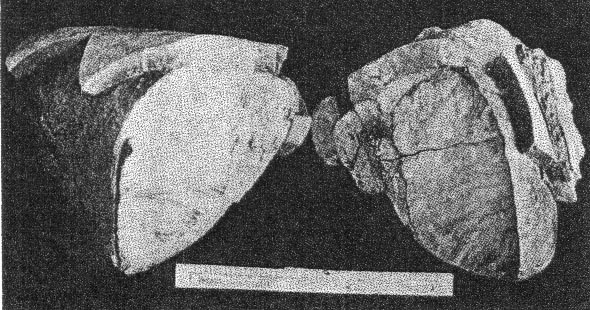
A. First stage of cookeite-quartz mineralization. White masses of microcline (best seen at lower right) becoming coated with cookeite and quartz. Buckfield.
B. Last stage of cookeite-quartz mineralization. Crusts of cookeite and quartz alone remain. Microcline and other older minerals have been completely removed. Buckfield. A great deal of cookeite is disseminated through and intergrown with the quartz of Class IV. Here it is usually pink or yellow, sometimes cream colored. It is on the whole earlier than the quartz, and appears to be undergoing replacement by quartz in a few places. One other occurrence of cookeite was observed at Buckfield. In this the isolated veins of lepidolite previously described have been partially altered into cookeite. Sometimes the secondary veins are completely altered, while the outer edges only of the main vein will be changed from lepidolite to cookeite. This is alteration in situ, cookeite resulting from hydration of lepidolite. Cookeite is usually considered an alteration product of either tourmaline or lepidolite. Examples of the alteration of tourmaline into cookeite are rare at Buckfield, but this change is well shown in specimens from Hebron and Mount Mica. Alteration, or hydration, of lepidolite into cookeite has just been described. But there is much cookeite at Buckfield and elsewhere in central Maine that cannot be accounted for by either of these processes. Bastin 28 says, in referring to cookeite: "Abundant as a coating in most of the pockets in the coarser pegmatites. Not an original constituent, but secondary and due to water deposition." But, much of the Buckfield cookeite is definitely earlier than the abundant and intimately associated quartz, which is in turn earlier than apatite and herderite. It would seem then that this cookeite is of hydrothermal origin. The low temperatures prevailing during this final period of hypogene deposition permitted the formation of cookeite rather than the less hydrous lepidolite.QUARTZ Quartz is the commonest mineral in Class IV. Some large masses resulting from this period of deposition consist of over ninety percent quartz. The crystals themselves are small, never exceeding two and a half centimeters in length. They are clear and absolutely colorless. Even the most massive specimens are extremely porous, as the crystals do not fit together very closely. These large quartz aggregates, consisting of a multitude of small individuals, are pink and yellowish in color, due to the presence of flakes of cookeite throughout the mass. The clear and colorless character and the small size of the quartz crystals usually distinguish them from those belonging in Class II. The latter are more often milky than colorless. When they are colorless distinction must be made on a basis of. association. The practically invariable presence of cookeite is a good criterion of later age. Sometimes the quartz crystals of Class IV project in parallel groups, from thin shelves. The latter are likewise parallel to each other and are usually composed of finer grained quartz. They may also contain cookeite, especially toward the center of the slab. Specimens illustrating this structure represent the last of a sequence of interesting events. In the first place a pocket was formed containing the typical minerals of Class II, such as lepidolite and cleavelandite. Further hydrothermal activity brought about the formation of lepidolite veins, connecting the lepidolite books. The roughly parallel structure of these veins may have been due to the platy character of minerals traversed, such as cleavelandite. Whatever the host it was soon removed by the dissolving activities of the ascending solutions. Lower temperatures and quantities of water brought about the hydration of the vein lepidolite into cookeite. Then silicification became dominant and the cookeite was partially or wholly replaced by quartz. From lepidolite to cookeite to quartz, then, is the history of some of these veins. During and after their last replacement they served as a base for many quartz crystals growing laterally into the open spaces between the shelves. Silicification as a final stage in pegmatite mineralization had a much greater development at Greenwood, and has been noted as an important phase at Auburn. APATITE
Apatite is unimportant among the minerals of Class IV at Buckfield. It is very clear, and pale green or light blue in
color. Crystals are characteristically tabular parallel to the base, and show the forms m(10 Class IV apatite is distinguished from earlier types by its habit and associations. It commonly lines cavities in the cookeite-quartz aggregates and where these cavities are tabular in shape the crusts on the opposite walls meet and simulate a vein. The pegmatites at Mount Apatite and Greenwood have produced many beautiful specimens containing apatite of this type. HERDERITE But few herderite crystals were found associated with the Class IV minerals. These were small, light brown, and clear. The herderite of Class II was translucent rather than transparent. Where found herderite was always associated with apatite in small openings in the cookeite-quartz masses. MINERALS OF CLASS V Most of the minerals in the last group occur as bristles, stalactites, crusts, or films associated with open spaces. They are without doubt secondary as in every case they consist of elements obtainable very near at hand and their method of deposition is typical of minerals formed by ground water activity. Many of the minerals themselves are characteristic of supergene deposition. Due to their secondary origin these minerals are found scattered throughout the pegmatite wherever the parent primary minerals are intersected by the zone of oxidation. Thus certain amounts of kaolin may be found where microcline, belonging to Class, I, lies close to the surface. Kaolin also results from the decomposition of spodumene, a Class II mineral. Cleavelandite, of the same class, is prone to alter into montmorillonite under near-surface conditions at Buckfield. The greatest variety of secondary minerals is found where the Class III minerals reach the zone of oxidation. The presence in the primary minerals of this class of MnO, P 2O5 and CO2 has been responsible for the formation of psilomelane, manganite, an unknown manganese phosphate, rhodochrosite, and dahllite.Owing to the scattered positions in the pegmatite of the secondary products no sequence of mineralization can be applied to the class as a whole. It is probable that more than one mineral was being deposited in different places at one time. A fairly definite sequence for the Class III secondary products is possible, however. Several specimens show psilomelane altering into manganite. Likewise manganite alters to an unknown manganese phosphate. Rhodochrosite is later than manganite, but dahllite was formed before it.KAOLIN A surprisingly small amount of the Buckfield feldspar is kaolinized. But spodumene was very susceptible to this alteration and fresh pieces of spodumene are rare. Many more specimens are found in which this mineral has been completely kaolinized. Usually the original structures of the spodumene are preserved, giving a true pseudomorph of kaolin after spodumene. Lepidolite veins formerly cutting spodumene crystals are found buried in a mass of kaolin in the pocket debris.Other minerals sometimes incipiently altered to kaolin are amblygonite, fairfieldite, cleavelandite, and topaz. MONTMORILLONITE Cleavelandite in the zone of oxidation is sometimes found partially altered to a pink, clay-like substance. This is one of the clay minerals, known as montmorillonite. The bright pink color of the fresh material pales upon exposure, probably due to partial dehydration. Under the microscope this material is seen to consist of an irregular aggregate of small scales or plates. The mineral is colorless in very thin plates, but becomes pink and opaque with an increase in thickness. Optical data are: Biaxial negative, 2V= almost 0, β = 1.513. Montmorillonite is a very indefinite species. The optical data above more nearly coincide with those given for leverrierite, 29 another hydrous aluminum silicate.PSILOMELANE Hard, black botryoidal crusts coating rhodochrosite proved to be psilomelane. This mineral is limited in its occurrence to the zone of weathering and even there it is quite subordinate to manganite, with which it is associated. The primary mineral from which psilomelane was derived was usually rhodochrosite. MANGANITE Manganite is one of the commonest of the secondary minerals. It is widespread in the surface zone, even forming a fine film on quartz and cleavelandite. Thicker deposits are found on the minerals actually undergoing alteration, such as rhodochrosite and lithiophilite. In a very few specimens manganite coated psilomelane, preserving the botryoidal character of that mineral. In this case it was quite evidently derived from psilomelane, but it is very doubtful that all the manganite passed through a psilomelane stage. In one instance the alteration about the edges of dahllite into manganite was observed.RED MANGANESE PHOSPHATE In a few specimens botryoidal surfaces of manganite (pseudomorphous after psilomelane) were coated by a thin, velvety, brick red crust. The latter very evidently resulted from alteration of the manganite, as the radiating fibers forming the rounded surface are red on that surface, but darken toward the interior, where they become indistinguishable from the black manganite. The fibers are extremely fine, and so soft and brittle that they powder immediately when an attempt is made to work with the material. The quantity present was insufficient for a chemical analysis. A bead test proved the presence of manganese, also a phosphate reaction was obtained with ammonium molybdate. Microscopic examination was hindered by the small size of the particles and their platy character. The mineral is biaxial and probably negative. The plates due to a good cleavage are perpendicular to X. Further optical data follow:
α = 1.71, 2V=large; It was at first thought that the mineral might be sicklerite, a hydrous lithium manganese phosphate. The indices of both lie between 1.71 and 1.75. Likewise, the pleochroism is somewhat similar for both. But comparison with the type sicklerite from Pala, California, brought out the following differences: 1. Birefringence greater for sicklerite. 2. Sicklerite gives good lithium flame, while unknown gives none. 3. Sicklerite readily soluble in nitric acid. Unknown but difficultly soluble. 4. Sicklerite is much harder. RHODOCHROSITE Ground water activity at Buckfield produced a third generation of rhodochrosite. In Class III this mineral appeared in masses, in Class IV in veins and druses, and in Class V in minute bristles or quills projecting out from the surfaces of earlier minerals. These much elongated crystals vary in color from white to bright yellow. A few may be seen in Plate II, B. The commonest primary minerals coated are rhodochrosite and reddingite. One small crystal of rhodochrosite has a pompadour of white secondary bristles. These project upward from about the top of a steep rhombohedron on the primary crystal. The secondary rhodochrosite fibres are parallel in crystallographic orientation to the host.These white or yellow quills of rhodochrosite are without doubt supergene in origin. They were deposited even after the manganite. In one specimen a somewhat spherical mass of manganite is covered with secondary rhodochrosite fibers like spines on a burr. DAHLLITE Dahllite, a calcium phosphate-carbonate, appears in yellowish green crusts coating various minerals of Class III. The coating is most striking when eosphorite is the host. Prismatic crystals of that mineral are often completely covered and the terminations rounded off, so that pseudostalactites result. Solution remnants of fairfieldite and rhodochrosite are similarly coated by crusts of dahllite. These may be seen on Plate III, A. Under the microscope the yellowish green crusts are seen to consist of parallel fibers normal to the surface. The mineral is uniaxial and negative, and the optical properties of the Buckfield material checked up quite closely with published data on dahllite. 30A minor occurrence of dahllite was noted in some of the rhodochrosite specimens. Instead of well-defined fibers dahllite here appeared in excessively minute yellowish particles replacing rhodochrosite. The fibrous dahllite seems to be merely a crust, resulting from ground water deposition on any handy mineral. On the other hand some dahllite replaced rhodochrosite, but without the production of fibers. At Mt. Mica dahllite was also observed as a dark green coating on eosphorite. The prismatic habit of the latter mineral produces pseudostalactites of dahllite similar to those at Buckfield. Certain specimens from Auburn in the Harvard mineralogical laboratories contained a mineral in small but well defined white rosettes which coated earlier minerals. Optical and chemical study proved the unknown to be francolite, a carbono-phosphate of calcium which is chemically very similar to dahllite, but contains fluorine and differs from that mineral in being biaxial. GREENWOOD The Greenwood deposit is located well up on the western flank of Noyes Mountain. A zigzag trail makes the steep ascent from valley to quarry floor. The slope of the ground is such that waste material which has been thrown over the dump is strewn down several hundred feet of mountain side. The country rock in which the pegmatite is intruded is a mottled green gneiss. The individual grains are small but the gneissic bands themselves are wide. The green color is due to the preponderance of pyroxene. Other important minerals visible in the thin section are chlorite, quartz, and oligoclase. The pegmatite dike cuts across the bands of the gneiss. Its outcrop runs along the brow of the hill for many yards, strikes N 60 W, and dips very steeply into the hill. The outcrop averages ten feet in width, but in one place it is much wider. Here an off-shoot of the main vein into the gneiss produces a total width for the pegmatite of about thirty feet over a short distance. The quarrying operations were mostly confined to this local swelling in the pegmatite. This was worked by an open cut extending from the steeply sloping ground surface back through the pegmatite to the hanging wall. Successively lower floors penetrated the mass deeper and deeper, and farther and farther in from the surface. When operations ceased in 1923 the floor at the hanging wall was about thirty feet vertically and horizontally from the original ground surface. The open cut is somewhat V-shaped, diminishing in width from about fifty feet at the top to fifteen feet at the floor. Two types of texture in the Greenwood pegmatite are very apparent. Away from the swelling the minerals, although coarse, are tightly intergrown. Specimens from these parts are massive with no open spaces visible. On the other hand the portions of the pegmatite within and neighboring the offshoot are extremely porous. Pockets are common and where these are lacking the material is replete with smaller cavities. The history of the mineralization at Greenwood is very similar to that at Buckfield. The first phase was the intrusion of the pegmatite magma, but at Greenwood there is no proof that this magma reacted with the wall rock. No xenoliths of gneiss were found in the pegmatite. The contacts are clean cut. The deposit is dike-like in form, and it was dike-like in its manner of intrusion. The solidification of the pegmatite magma produced one group of minerals. Here occur feldspar, quartz, and other minerals making up the main mass of the pegmatite. As at Buckfield, liquids discharged during the crystallization of successively lower portions of the magma flowed upward, dissolving and depositing as they did so. They did not confine themselves to the outlines of the original pegmatite but extended their activity out into the gneissic country rock. This produced the bulge or swelling in the dike previously described. But two phases in hydrothermal activity may be recognized at Greenwood. The first of these was exactly the same as at Buckfield. This was a silica-sodium-rare element phase, producing such minerals as quartz, cleavelandite, lepidolite, tourmaline, caesium beryl, etc. Buckfield's Class III, resulting from a lithium-manganese-phosphorus phase, is lacking. Not a mineral of that suite, with the exception of amblygonite, has been found at Greenwood. The fourth class, however, was extraordinarily well developed here. At Buckfield this was a lithium-silica phase. At Greenwood phosphorus must be added to the list, for apatite is present in abundance along with cookeite and quartz. Supergene activity produced but few minerals. The most interesting of these is kaolin formed from amblygonite in nodules. MAGMATIC PHASE The magmatic phase of the Greenwood pegmatite produced much the same mineral association as at Buckfield. The major constituents are white and pink microcline in anhedrons under a meter across and gray to colorless quartz. Large books of muscovite up to sixty centimeters across and a centimeter thick are common at Greenwood but were not found at Buckfield. Transparent to opaque green beryls are found but are less abundant than at Buckfield. The largest crystal found in 1923 was fourteen centimeters across and ten long. Black tourmaline and dark green manganapatite are similar in character and occurrence to those at Buckfield. Small red garnets are concentrated in layers parallel to the walls of the pegmatite as they commonly are in other such deposits. A thick layer is next the footwall; a thinner layer below the hanging wall has garnet, black tourmaline and microcline. Arsenopyrite in widely scattered small anhedrons is rare. FIRST HYDROTHERMAL PHASE The magmatic phase was succeeded by two hydrothermal phases. The first of these was characterized by large amounts of silica, resulting in the formation of many quartz crystals; sodium, which combined with silica and aluminum to form albite; and several rare elements such as glucinum, boron, caesium, lithium, columbium, which entered into a variety of minerals. As at Buckfield cavities were formed by dissolving activity on the part of the ascending solutions. One pocket was three meters long and several pockets a third of a meter to a meter in diameter were found. Many of the cavities were completely lined with hydrothermal minerals, in others massive quartz formed a part of the wall. Very little difference exists between Greenwood and Buckfield in regard to the minerals produced during this period. At both localities quartz, cleavelandite, and lepidolite predominate over the other minerals of this class. Of the minor minerals tourmaline and spodumene were more abundant at Greenwood than at Buckfield. On the other hand some of the rarer minerals at Buckfield do not appear at Greenwood. QUARTZ A great variety of quartz crystals, and some magnificent specimens, have been quarried at Greenwood. These have been found either lining the cavity walls or loose in the pocket sand. Crystals vary in length from one to thirty centimeters. The large crystals are usually milky, the smaller ones clear or smoky. Many small pockets were barren save for one or two small clear or smoky quartz crystals. In the larger pockets groups were the rule. Some of these consist of several odd sized crystals in irregular orientation. Others, of truly spectacular appearance, consist of a parallel aggregate of smoky or milky quartz crystals (Plate VII, B). Besides the uniform orientation, these individual crystals are remarkably consistent in size. They vary from one to four centimeters in length, over an entire specimen, but any two adjacent crystals are usually quite similar in size. These parallel groups may be frozen on to massive quartz of the pocket wall, or they may cluster on a single large quartz crystal. In the latter case crystallographic directions in the individuals of the aggregate are parallel to similar directions in the host. In some. instances the large crystal is incompletely developed on one side, and a multitude of smaller crystals have so arranged themselves as to tend to complete the crystal form.The separate crystals in a cluster usually merge together on prism faces. Sometimes the groups are tabular, due to addition in one plane only. At other times growth has been in all directions, and striking turret-like forms are the result. There is some evidence that the earlier crystals to form were milky in color, and the later ones smoky. In some instances smoky crystals give way to milky crystals as one penetrates into the cluster. One slab two feet long is covered by a host of small parallel quartz crystals. Those on the outside are smoky, but farther in they become milky. The ascending solution deposited quartz over rather a long period as shown by the succession of clusters on many specimens. OTHER MINERALS The cleavelandite at Greenwood does not differ from that found at Buckfield. It is typical of early hydrothermal activity at both localities. Large masses were found in and about the pockets in the pegmatite. Lepidolite was formed during one period only at Greenwood. Here it occurs either in beautiful lilac colored books up to one centimeter wide and an equal thickness, or in massive aggregates of fine plates, lavender, white, or even pale green in color. Some of these aggregates were thirty centimeters or more through. Both types of lepidolite are commonly associated with cleavelandite. Tourmaline likewise has two modes of occurrence. It is sometimes found in green crystals radiating rather irregularly from the pockets. Although fairly clear these crystals are never gem worthy. Most of the individuals are from five to eight centimeters long, but an occasional one is twice that length. Later silicification has in most cases partially destroyed the crystals. In the second occurrence tourmaline appears in green transparent crystals. When found these are usually loose in the pocket sand. Some are doubly terminated and thinner than a needle. As at Buckfield many of the pocket tourmalines are gem worthy. The largest specimen of spodumene collected at Greenwood is fifteen centimeters wide and thirty long and this is but a fragment of a larger piece. The mineral is decidedly replacive, exhibiting an irregular contact toward the older magmatic minerals. It is pale green, and some pieces are translucent. Kaolinization is incipient only.
PLATE V
A. Amblygonite nodule. White core is amblygonite, surrounded by varicolored crusts of kaolin. Greenwood.
B. Amblygonite nodule sawed in two. Note fairly sharp contact between amblygonite core and kaolin rim. Greenwood.
FIG. 4. Amblygonite crystal.
Amblygonite occurs in very striking forms. One crystal found by Mr. Noyes and shown in FIG. 4 is a complete individual
15 cm. long, 8 wide and 5 thick. It is flattened parallel to the dome h( Cassiterite was found sparingly, in part in irregular masses or stout pyramidal crystals embedded in cleavelandite, in part in crystals of unusual form attached to a surface of cleavelandite plates. One of these crystals is shown in FIG. 5 and is remarkable in showing practically only a ditetragonal pyramid as its bounding form, doubly terminated and as symmetrically developed as a model. The largest of these crystals was not more than four millimeters across. The same rare form, μ (766) is shown in a somewhat similar figure by Jeremejew, of cassiterite from Siberia reproduced in Goldschmidt Atlas der Krystallformen Vol. IX, plate 89, figure 227.
FIG. 5. Cassiterite Other minerals of the pocket zone at Greenwood are pale green to colorless beryl, mostly deeply etched, rare embedded crystals of dark green gahnite or zinc spinel, columbite in masses up to a centimeter across, and herderite, bertrandite and hamlinite, the last three in minute amount only, in implanted crystals. SECOND HYDROTHERMAL PHASE The second and last hydrothermal phase was characterized by enormous amounts of silica, with smaller amounts of lithium and phosphorus. As a result quartz is the outstanding mineral. Cookeite and apatite are common, but are quite subordinate quantitatively. The minerals resulting from this period of activity are confined to the swelling in the pegmatite, and in many portions of this zone their deposition has been accompanied by the absolute obliteration of earlier minerals. Cookeite was deposited with quartz, but apatite is distinctly later. Its single crystals and druses rest upon quartz and cookeite. QUARTZ AND COOKEITE Many of the quartz-cookeite specimens from Greenwood are indistinguishable from those found at Buckfield. The quartz crystals are small and colorless or milky. Cookeite appears in scales and flakes disseminated through the quartz aggregates. It varies in color from white to pink. The first quartz to be deposited formed as druses on pre-existing minerals. If crystals were available these were completely coated by a layer of small remarkably uniform quartz crystals. At other times this coating was deposited on mineral aggregates, or as a lining on the walls of solution cavities. Deposition was accompanied and followed by intense dissolving activity. In every case the material coated was removed. If the host was an euhedral crystal the coating retained the outline of that crystal. Sometimes, instead of being coated, the original mineral was completely replaced by fine grained quartz and cookeite. These two types of pseudomorphs are described and discussed under a separate heading. But, if the host was an ill-defined aggregate merely an irregular crust resulted from its removal. Many specimens of both pseudomorphs and crusts were found in the cavity zone of the pegmatite. Quartz and cookeite continued to be deposited after much of the pre-existing material had been dissolved and removed. Having a great abundance of space at its disposal this quartz merely piled up on itself, its individuals assuming little or no similarity in orientation. It is this type of aggregate that gives certain portions of the pegmatite their high porosity. CHARLES PALACHE AND K. K. LANDES The quartz pseudomorphs taken from the Greenwood ledge in numerous specimens by Mr. Noyes when the large pockets were first opened are among the most interesting minerals which it yielded. They are of two types and both were described by Warren,31 the one as a pseudomorph after phenacite, the other as after topaz. It was largely in the hope of solving the riddle of their nature that ownership of the Greenwood ledge was secured for the Harvard Museum and the later excavation there carried on. Many new specimens of both types were then secured but none approaching the beauty of the early groups; nor was the hope of finally determining their nature fulfilled although a new interpretation is offered on a later page. According to Mr. Noyes the large pocket (see pages 363 and 400) was completely encrusted either with quartz crystals or with the hollow pseudomorphs or both. He saved many single specimens and large slabs showing groups such as are reproduced in Plate VI. During our quarrying hundreds of specimens showing broken or imperfect pseudomorphs of the same kind were thrown over the dump. They vary in size from a centimeter or less in diameter to more than half a meter. Whatever the primary mineral may have been it was certainly developed in very great abundance in all the cavernous parts of the ledge. Of the embedded, solid pseudomorphs only a few have been found. The largest, described by Warren and now in the Harvard collection, has a diameter of about thirty centimeters and weighs twenty eight pounds. The two types of quartz pseudomorphs, one due to replacement and the other to incrustation, also differ from each other crystallographically. Two minerals, then, played the part of host. No traces other than external form remain of either of these original minerals, but some of the pseudomorphs contain cleavelandite, which was evidently replacing the missing minerals. Both types of pseudomorph have been found on the same specimen. It is evident that the original minerals were associated together and preceded cleavelandite in formation. However, their euhedral form and aggregate arrangement point toward deposition in open spaces, rather than crystallization from the primary pegmatite magma. The conclusion is that the original minerals of the pseudomorphs were deposited rather early during the first hydrothermal period. Examples of the replacement type of pseudomorph are shown in Plate VI, A and will be described first. These are composed of fine grained quartz and cookeite with an occasional remnant of cleavelandite. Crystal faces are plentiful and definite. Five specimens of this type of pseudomorph have been found, and the same groups of faces occupy each. Contact Measurements of Quartz Pseudomorphs.
PLATE VI
A. Quartz pseudomorphs after analcite (?) showing the forms a(100) and i(211). Embedded in fresh microcline.
B. A group of hollow quartz pseudomorphs after heulandite (?). Also parallel quartz crystals grouped on two large milky quartz crystals. Greenwood. The form is a cube, with each corner truncated by three faces of the trapezohedron (211). The angles check as closely as could be expected when one considers the rough surfaces involved. The maximum error is about two degrees. When the form is set up correctly its character is unescapable. The smallest cube among these pseudomorphs is two inches, the largest is nine. The latter specimen contained three of these nine-inch cube faces, with the corner formed by their junction truncated by three smaller trapezohedron faces. Other trapezohedron faces marked other corners. Under the impression that the form was rhombohedral Warren32 described the original mineral as phenacite, but the definite isometric character of the pseudomorph rules out this mineral. The most plausible guess as to the original mineral is analcite. This is supported by the following facts: 1. It is the only known mineral of exactly similar habit. One crystal of analcite in the Holden collection from the Tyrol is a perfect model of the pseudomorph. On page 596 of Dana's System of Mineralogy a drawing of an analcite crystal (Fig. 3) from the Cyclopean Islands (near Catania, Sicily), could just as well be labelled a pseudomorph from Greenwood. 2. The original mineral was deposited during the first hydrothermal period when we know the solutions were sodic. 3. Although hydrous and a zeolite, analcite is known elsewhere as an igneous mineral, so temperature considerations cannot. rule it out here. Points unfavorable to the analcite hypothesis are: 1. The large size of the pseudomorphs. Nine inch cubes as opposed to three inch crystals from the Tyrol. 2. Analcite is more often associated with rocks of intermediate or basic character. No other possibilities suggest themselves; the cube precludes garnet, the trapezohedron precludes fluorite. The second type of pseudomorph resulted froze incrustation by drusy quartz, followed by the solution and removal of the host. Pictures of this type are given on Plate VI, B. Some are hollow, others are partially filled with later quartz or earlier clevelandite. The form is prismatic, terminated bluntly by faces at ninety degrees to the axis of elongation. The end faces, giving the cross section of the form, are sometimes diamond shaped, at other times six-sided. The apparent orthohombic symmetry in many of the forms suggested to Warren that the original mineral was topaz.33 Other specimens, however, appear to have but one plane of symmetry, and that at right angles to the axis of elongation. If that is true the pseudomorphs are monoclinic, and consist of side pinacoid, base, and positive and negative orthodome. Attempted measurements of the angles produce a variety of results. Contact methods only are available and any respectable degree of accuracy is prevented by the extremely rough nature of the surfaces involved. However, even with the eye, a rather wide divergence of angles may be noted. The acute angles about the end faces of the different pseudomorphs vary between 28° and 57°. Any theory as to the original mineral will have to allow for this wide variation. The suggestion is made that the host for the incrustation was heulandite, elongated parallel to the b axis. Arguments in favor are: 1. Crystallographic similarities. The side pinacoid, base, and positive and negative orthodomes are very important forms in both. In some of the best developed pseudomorphs the angles match very closely with those given for heulandite which is often pseudo-orthorhombic in form. Oscillatory combination of orthodome faces known on heulandite would well account f or the variety of angles in the pseudomorphs. Furthermore the inside of the crust is often striated parallel to b, as it would be if the crystal faces on which deposition took place were so striated. 2. The close association with another zeolite, analcite. Unfavorable points are: 1. Large size of pseudomorphs. The side pinacoids on the largest specimens measure five to ten centimeters, and the b axis is 15 centimeters in length. 2. Elongation parallel to b is an unusual habit for heulandite. 3. This mineral occurs more commonly associated with less acidic rocks. Another possibility is bertrandite, a hydrous beryllium silicate which is found associated with beryl in pegmatites. Its crystal form is somewhat similar, but its individuals are usually very minute. Still a third possibility is feldspar, in elongated crystals with side pinacoid, base, and negative orthodomes.34
PLATE VII
A. Apatite lining a cavity dissolved from fresh microcline.
B. Quartz showing parallel growth of clear smoky quartz on an older milky quartz crystal of more prismatic habit. Greenwood.
APATITE The apatite crystals are purple and clear. They appear either in large isolated crystals or in druses, composed of much smaller individuals. The largest of the isolated apatite crystals measure two centimeters in diameter and a little less in length, but the most are only about half this dimension. They appear scattered through the quartz-cookeite masses, but always resting on, rather than embedded in, these minerals. Two or three such crystals, purple and clear, nested in a small vug walled by scores of transparent quartz crystals, make a very pretty picture. But the most spectacular occurrence of apatite at Greenwood was as a drusy lining on the walls of pockets. The largest pocket so lined was pod-shaped, forty centimeters long and averaged seven centimeters in width. A cavity of similar shape but only fifteen centimeters long adjoined. Nearby were still other pockets of more irregular shape, but likewise lined with drusy crystals of apatite. The pockets of the latter type are very obviously solution cavities in fresh microcline. One of these is shown on Plate VIl, A. The pocket walls which are lined with apatite present a rare sight. They appear as a continuous purple mass, sparkling from a hundred points, as the individual crystals of the druse catch the light and flash it back. SUPERGENE PHASE The ground water solutions at Greenwood had little to work on that was susceptible to their activity. No suite of fairly soluble manganese phosphates was present to give a wide range of secondary products as at Buckfield. Supergene mineralization was practically confined to the kaolinization of feldspar and amblygonite.In the case of feldspar the masses of that mineral near the surface, or adjacent to the highly porous portions of the pegmatite, have been altered to some degree. At times montmorillonite resulted from this decomposition. The nodules of amblygonite appear to have been converted into kaolin by concentric zones working inward. In the nodules pictured (Plate V) the outer crusts are kaolin and sericite, but the core has not as yet succumbed to this alteration. In other instances the center of the nodule is white and compact, with a conchoidal fracture. Here kaolinization has been more complete, A green crust also surrounds the kaolin core. Analyses by Miss Vassar of the green crust and the white core follow:
CONCLUSIONS The detailed study of the pegmatites at Buckfield and Greenwood has shown that at these two localities the original crystallization of the pegmatite magma resulted in an ordinary coarse grained pegmatite made up chiefly of microcline, microperthite, and quartz and containing subordinate apatite, green beryl, black tourmaline, lepidolite, biotite, garnet, arsenopyrite, and amblygonite. After the crystallization of the pegmatite the residual solutions moved upward through the pegmatite, dissolved and replaced the original minerals and deposited new minerals. The first solutions deposited chiefly quartz, clevelandite, lepidolite, light colored beryl, and tourmaline, spodumene, apatite, and other rare minerals. Later solutions deposited rhodochrosite, amblygonite, and rare phosphates. The last hydrothermal solutions deposited chiefly lepidolite, cookeite, quartz, apatite, and herderite. A study of the literature and a brief field and laboratory examination of the pegmatites at Mt. Mica and Auburn, Maine, show that these pegmatites too were formed in successive stages, similar to those of the Buckfield and Greenwood pegmatites. The history that can be worked out is no doubt fragmentary for all the deposits and in some more stages can be recognized than in others. At Mt. Mica the second hydrothermal stage was not found but at Mt. Apatite, near Auburn, all three hydrothermal stages were recognized. NOTES * Presented as part of the requirement for the degree of Doctor of Philosophy in the Division of Geology at Harvard University. 1 Bastin, E. S. Geology of the pegmatites and associated rocks of Maine. U. S. Geol. Surv. Bull. 445, 1911. 2 A very brief preliminary report on this deposit by Dr. Charles Palache was published in the March, 1924, American Mineralogist. 3 Delesse, A., Sur la Pegmatite de l'Ireland, Bull. Soc. Geol. de France (2), 10, p. 570, 1853. Quoted by Kemp, The Pegmatites, Econ. Geol., December 1924. 4 Brögger, W. C., Die Mineralien der Syenitpegmatitgänge der Südnorwegischen Augit- and Nephelinsyenite, Zeit. Kryst. Min.., 16, 1890. Sections on genesis translated by Evans, Can. Rec. Sci., 6, pp. 33-46, 61-71, 1894. 5 Crosby, W. O. and Fuller, M. L., Origin of Pegmatite, Am. Geol., 19, p. 172, 1897. 6 Bastin, E. S., Geology of the pegmatites and associated rocks of Maine, U. S. Geol. Surv. Bull., 445, p. 32, 1911. 7 Loc. cit., p. 71. 8 Loc. cit., p. 86. 9 Lacroix, A. Mineralogie de Madagascar, Vol. 2, p. 310, 1922. 10 Laubmann, H. and Steinmetz, H., Phosphatführende Pegmatite des Obelpfalzer and Bayerischen Waldes, Zeit. Kryst. Min., 55, 584, 1915-1920. 11 Loc. cit., p. 88. 12 Bowman, H. L., Occurrence of Minerals at Haddam Neck, Connecticut, Mineralog. Mag., May 1902. 13 Lacroix, A., Mineralogie de Madagascar, Vol. II, p. 304, 1922. 14 Verbal communication. 15 Bastin, E. S., Geology of the pegmatite's and associated Rocks of Maine, U. S. Geol. Surv, Bull., 445, p. 13, 1911. 16 Schaller, W. T., Mineralogical Notes, U. S. Geol. Surv., Bull., 490, p. 95,1912 17 Brush, G. J., and Dana, E. S., Am. J. Sc., 16, pp. 33-46 and 114-123, 1878. 18 A. Lacroix, Mineralogie de Madagascar, Vol. 2, pp. 292 and 293, 1922. 19 Laubmann, H., and Steinmetz, H., Loc. Cit., p. 570. 20 Amblygonite is as often positive as negative, and shows a wide range of indices, according to optical studies by Helge Backlund, Amblygonite at Üto, Papers of Mine. Inst., Stockholm University. Vol. I. No. 4, 1918. 21 Brush and Dana. Loc. cit., p. 67. 22 Graton, L. C., Gold and Tin Deposits of the Southern Appalachians, U. S. Geol. Surv., Bull. 293, p. 38, 1906. 23 Larsen, E. S., The Microscopic Determination of the Non-opaque Minerals, U. S. Geol. Surv., Bull. 679, p. 72, 192 1. 24 Drugman, Julien, On Childrenite from Crinnis Mine. Cornwall, and Eosphorite from Poland, Maine. Mineral. Mag.,1915, pp. 193-201. The Poland eosphorite contained 5.55% FeO and showed the same forms as are found at Buckfield. 25 Loc. cit. p. 57. 26 Jahrb. fur Mineralogie, p. 370, 1879, No. 1, p. 185, 1885. 27 Optical data and sketches of cookeite by R. W. Goranson. 28 Bastin, E. S., Geology of the pegmatites and associated rocks of Maine U. S. Geol. Surv, Bull. 445, p. 16, 1911. 29 Larsen, E. S., The microscopic determination of the non-opaque minerals, U. S. Geol. Surv, Bull. 679, p. 245, 1921. 30 Larsen, E. S., Loc. cit., p. 196. 31 Warren, C. H. Mineralogical Notes. Am. J. Sci., vol. 6, 119, 1898. 32 Loc. cit., p. 120. Warren's figure (idealized for phenacite) may be found also in Appendix I, Dana, System of Mineralogy, p. 53. According to our interpretation the faces lettered a are the cube faces; all the others are faces of the trapezohedron. The rhombohedral aspect of the crystal is due to setting up the cube with a diagonal as vertical axis. 33 Loc. cit., p. 121. 34 Since writing this discussion of the original nature of these pseudomorphs a suggestion has been made by Dr. W. F. Schaller which should be recorded here. He pointed out that pollucite was an isometric mineral not considered in the discussion and very likely to have occurred in the pegmatite. It has been found in great abundance in a neighboring pegmatite in Buckfield, (Dudley's Ledge) although not in crystals. Dana records the occurrence of crystals in the Elba pegmatites with the same forms, a, (100) and n, (211) as are present on the psuedomorphs but as these have never been figured, the habit is not known. It is obvious that pollucite is a much more likely mineral to have been formed in this pegmatite than analcite. The writers also desire to place more emphasis on the possibility that the orthorhombic or monoclinic pseudomorphs might have been bertrandite. This mineral has never been found in crystals of more than a fraction of an inch in diameter but size is the most variable element of the pegmatite minerals.
|