| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
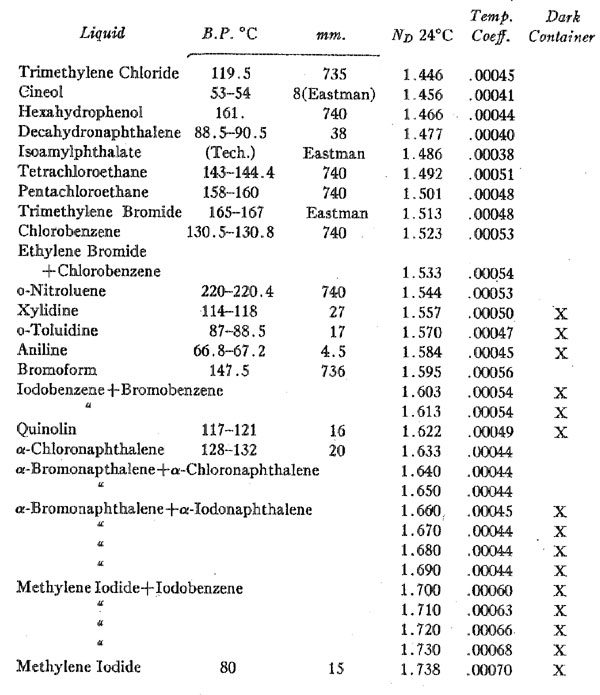
Volume 14, pages 482-483, 1929 A SET OF THIRTY IMMERSION MEDIA R. C. EMMONS, University of Wisconsin. During the course of some experiments recently completed it was necessary to measure the optical constants of a very large number of liquids and to study many more whose constants are given in tables of critical data. Having on hand then a considerable amount of data and material, it was deemed worth while to select a set of immersion liquids which might serve the petrographer better than those commonly used today. Objections to standard immersion media are many and well known. Probably one of the greatest drawbacks is the extent to which these media change in index even over a short period of time. Essential oils commonly used such as cedar oil, clove oil and others are natural mixtures from which the lighter constituents evaporate, leaving a gummy residue. Others in standard sets are manufactured mixtures which change index even more rapidly. Such liquids when purchased are correctly marked in index to a round figure. However, the sets used by nearly all petrographers have corrected figures of successive dates on the bottles, and these are not in convenient round figures. Consequently it is almost essential to measure each liquid on the refractometer each time it is used. Since the index of any liquid depends upon the temperature, it becomes necessary to work at a uniform temperature or to know what the temperature is. The difference in indices of commonly used liquids ranges for a degree centigrade from 0.0003-0.0007. These constants are given for the liquids listed below. The liquids listed in the table are so far as possible single compounds. Evaporation does not, therefore, change the index. For the liquids of higher index it is necessary to use mixtures since liquids of high index are rare. The mixtures chosen are made of very similar liquids whose boiling points are close together. Evaporation therefore takes place in both liquids of the mixture at almost the same rate of speed. The change in index in these liquids due to this cause is slight, and seems to be at a minimum. They will be replaced by single compounds if suitable ones are found. There seems, however, to be little hope of finding suitable liquids of higher index and constant boiling point. None of the liquids have indices which are represented by round figures nor is the interval between each pair of liquids exactly .010. Nor is this true of standard sets after a short period of time. The greatest interval is .014 and the least .006. The advantages offered by this set seem more than to offset these slight disadvantages. The liquids1 are listed in the following table together with their boiling points, indices at room temperature and temperature coefficients.
NOTE 1 All of these liquids can be obtained from the Eastman Kodak Co., Rochester N. Y., or in sets ready for use from Dr. C. W. Muehlberger, Service Memorial Institute, Madison, Wisconsin.
DVB - This article is primarily for historical interest. Immersion fluids that are much safer are now available. (2003) |