| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
Volume 14, pages 207-221, 1929
THE ORIGIN OF THE ZINC DEPOSITS AT FRANKLIN AND STERLING HILL, NEW JERSEY W. A. TARR, University of Missouri. The origin of the zinc deposits at Franklin and Sterling Hill, New Jersey, is a question of great interest to the student of mineralogy and ore deposits. Several excellent summaries of the geology, with its numerous problems of structure, petrology, and metamorphism, and of the mineralogy and paragenesis of the ores have appeared in recent years.1 Each of these authors has presented his interpretation of the origin as a result of his studies of the area. There is general agreement as to the fundamental elements in the geology of the area and the occurrence and mineralogy of the ores, but the authors of each of the last four papers published on the subject have presented evidence favoring as many different methods of origin. The very uniqueness of the mineralogy of the zinc ores: franklinite, willemite, and zincite, (unlike any other zinc deposit in the world) makes their origin an entrancing problem. The origin of the great group of rare minerals developed by the intrusion of the pegmatites into the ores or their vicinity has been correctly interpreted, the writer believes, by Spencer, Ries and W. C. Bowen, and Palache. The magnetite deposits cannot be ascribed to the same source or time as the zinc deposits. They are probably of a later date. The recent excellent paper by Professor Palache, giving a paragenetic classification of the minerals of this region is accompanied by an expression of his theory as to the origin of the deposits. The writer, who is in essential agreement with Professor Palache's theory, had considerable hesitancy in writing this. He had, however, developed the same theory independently some years ago and has been teaching it to his classes in ore deposits. Somewhat the same theory was independently developed also by R. H. Rastall2 of Cambridge University and is given in his volume on "The Geology of the Metalliferous Deposits." The writer discussed the origin of these minerals with Dr. Rastall in 1925, and in August of that year visited and studied the mines for a few days. The objective in writing this paper is to present a discussion of the simple chemical and mineralogical method by which the present mineralogy of the deposit was brought about, probably something like what Palache had in mind but which he did not develop. The writer will not review in detail the geology of the area in this paper as it has been adequately presented in Palache's paper, so recently, as well as in other papers cited. This paper will present the writer's theory as to the composition of the original ore body, its change into an oxidized body, and its final conversion into the present ore body, and the evidence for the theory. CHARACTER OF THE ORIGINAL DEPOSIT The present zinc ore is a folded tabular body in the Franklin limestone (marble) of pre-Cambrian age. The limestone is generally believed to be a part of, or equivalent in time to, the Grenville series. No other known zinc ore body consists of a group of minerals like those in this deposit, i.e., franklinite, willemite (and tephroite), and zincite. These minerals do not occur in any type of deposit directly connected with igneous rocks, or in those not connected with them. The New Jersey deposit has been metamorphosed, regionally, subsequent to the deposition of the ores. We are thus led to conclude that the deposit which became the source of this unique ore body was similar to the hundreds or even thousands of zinc deposits scattered over the earth's surface, and which constitute our chief source of zinc. In these common deposits, the chief zinc mineral is sphalerite, oxidized, in greater or less degree, to smithsonite or hemimorphite or, rarely, willemite. It is associated with varying amounts of other sulfides including pyrite or marcasite, galena, and chalcopyrite. Like the sphalerite, these sulfides undergo alteration in the zone of oxidation. A great many gangue minerals occur with these zinc ores. These include carbonates, sulfates, oxides, and silicates. Some deposits are notably simple in their mineralogy and others are very complex. Using the metals found in the New Jersey deposit (the ratio of Zn: Fe: Mn is 3:3:1) as a basis for deducing the mineralogy of the original ore body, it is believed that that deposit consisted (in the order of their probable abundance) of sphalerite, pyrite or marcasite, calcite, and rhodochrosite. This represents a maximum of simplicity in mineralogy, but it must not be thought that other minerals might not have been present. Quartz or an unknown silicate may have been present, and a manganiferous siderite may have been present. If galena were present in the original deposit, the modern ore body was separated from it during oxidation. Galena, however, is not believed to have been present. It is possible that the ore solutions may have introduced some manganese into the calcite in the limestone surrounding the ore body, but this occurred more probably during the period of oxidation. This aggregate of minerals, sulfides and carbonates, was deposited in the Franklin limestone (probably while it was a limestone) by solutions from an unknown source. It is not improbable that some part of the Pochuck gneiss may have been the source rock, but such a suggestion is only conjectural. The simple mineralogy suggests a deposit near the surface. OXIDATION OF THE ORIGINAL SULFIDE DEPOSIT The next step in the development of the zinc deposits was the oxidation of the postulated primary ore body. This would occur if the ore body was brought to or near the surface. The oxidized products, again, were relatively few. The sphalerite was converted into the carbonate (smithsonite) and the hydrous silicate (hemimorphite). The iron sulfide (or sulfides) was oxidized and became hematite or limonite. The rhodochrosite was probably oxidized, in part at least, to manganite, pyrolusite, and braunite. The hydrous zinc silicate, hemimorphite, was more abundant in the oxidized deposits than was smithsonite. The deposits at Moresnet, Belgium,3 and at Granby, Missouri,4 are similar types, as both occur in limestone. Hemimorphite occurs in more complex ores also, as at Leadville, Colorado,5 and in a small mine in southwestern New Mexico described by Blake.6 The source of the silica that entered the hemimorphite is of interest. Had the original ore body been near an igneous rock, as at Leadville and in the New Mexico deposits, a source would have been at hand. Likewise, the deposit at Granby, Missouri, had an adequate source in the chert of the associated limestone. Rastall7 suggests that the original rock at Franklin might have been a siliceous limestone, but this does not appear to have been possible, for the present limestone or marble is very pure, containing, for the most part, less than one per cent of silica, and rarely reaching 2.5 per cent. Quartz does not appear as a gangue mineral of the present ores. That it may have been present in the original ore body is entirely possible, but if so, it has been converted into hemimorphite (and braunite). The most probable source for the silica appears to have been the weathered mantle rock, or an overlying, or nearby, shale or sandstone. Minor amounts may have been derived from the limestone itself, and, also, from quartz or silicates in the original ore. It is unlikely that there was so complete a balance between any original quartz and the zinc as to leave no quartz in the ores. Furthermore, there was a deficiency of silica, as is shown by the presence of smithsonite. It is probable that the silicate formed after the carbonate (possibly in part replacing it), although if silica were in the oxidizing solutions, the silicate might be formed first or at the same time. The hemimorphite in the Moresnet deposits is in limestone. Some of the silicate ore bodies in those deposits are very large, hence the amount of original silica must have been considerable. Its source is unknown; very probably it came from the limestone or from some nearby slates. The hemimorphite of the New Jersey deposits contains about six or seven per cent of manganese oxide (MnO), but is essentially free from ferrous oxide, a fact characteristic of the smithsonite also, as noted below. Zinc carbonate is more insoluble than calcium carbonate, and is, therefore, a common alteration product of sphalerite, especially in limestone. Both manganese and iron are isomorphous with zinc in the carbonate and may replace it in any amount. The following analyses are quoted from Doelter8 to show the wide range in composition of smithsonite in one area. The table gives the composition of the oxidized zinc deposits in Aachen, Prussia, (near to and similar to those at Moresnet, Belgium).
The smithsonite in the deposits at Franklin and Sterling Hill is very low in ferrous iron, as will be pointed out later, but contains about six per cent of manganese oxide. The iron sulfide of our original ore body was oxidized to hematite and limonite, as has been said. The sulfate radical, thus freed, may have been a factor in introducing the manganese into the calcite, for manganese is readily transported as the sulfate. A significant feature of the oxidation and hydration of the iron sulfide was the completeness of the process. As there is practically no ferrous oxide in any of the present ore minerals, all the iron must have been oxidized. Pyrolusite and manganite were formed but apparently not in abundance. Braunite appears to have been formed also. The amounts of the different manganese oxides are difficult to estimate. There is no evidence that there were any deposits of the so called "tallow" or zinciferous clays in connection with the oxidized deposits. Aluminous minerals do not occur in the ores; undoubtedly, if there had been any originally, they would still be present, as such minerals are very difficult to remove.
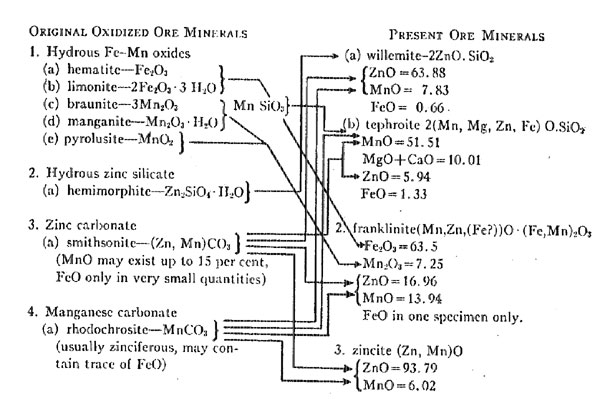
The mineralogy of the oxidized ore body (exclusive of the calcite), in the order of abundance, is believed to have been as follows: Other minerals may have been present but it is not necessary to assume this, because the mineralogy as outlined above is adequate to fully account for the origin of the actual minerals in the present ore body. FORM AND POSITION OF THE OXIDIZED ORES The tabular form of the present ore bodies is, no doubt, similar to the shape of the original bodies. The curved or hooked shape is due to subsequent folding. Aside from the thickening the deposits have undergone as a result of folding and flowage, they are essentially as originally deposited. Thus the originals were, we assume, tabular bodies. Two probabilities exist as to their original position. One is that they were residual masses on the surface, but resting upon and replacing the underlying limestone. The other is that they were tabular or bedded deposits wholly within the limestone. Examining the possibilities of the first premise, we find that the deposit must have been wholly on the surface and unassociated with clays, for there are no aluminum silicates in the ore proper (there were some associated with the metamorphism due to the pegmatites, but they were derived from the pegmatites). Residual iron and manganese ores resting on the surface, as we find them today, illustrate the probable original form and position of the zinc ore body, but such present deposits, rarely, if ever, are entirely free from clay materials. This objection in the matter of clay would apply to the possible derivation of the zinc ores from a deposit such as the secondary accumulation of hemimorphite, "smithsonite, zinciferous clay and iron and manganese hydrates" at Sterling Hill, described by Palache. There can be no question that this combination of minerals would be a possible source for a franklinite-willemite-zincite-ore, if it were not for the clay. Had clay been originally present, some evidence of it would undoubtedly still be found in the ores, for it is almost impossible to remove aluminum silicates from a deposit. The zinc minerals would have been removed long before the aluminum silicates were. It seems necessary, therefore, to assume that the oxidized material was a tabular mass within the limestone, because if the original, primary sulfide mass was altered in place or transported and replaced a bed within the limestone, the change would have taken place by solutions free from any aluminous substances. If the oxidized ore body was due to oxidation in place, the form and position of the original body must have been tabular. If it was due to replacement by solutions, the structural or lithological features of the rock controlled the form and position. The ore body may have developed along a fissure of some type, or along some readily replaceable bed, either of which possibilities would (or could) permit the development of the tabular character, of the deposit. The branching of the bed at Sterling Hill could be readily explained as replacement along a branching fissure. Another point favorable to the idea that the oxidized ore body was wholly within the limestone is the fact that the present ore bodies at both Mine Hill (Franklin) and Sterling Hill are entirely surrounded by the Franklin limestone. A surface deposit would, of necessity, have to be infolded to be so surrounded. ORIGIN OF THE PRESENT ORE MINERALS The conversion of the carbonates and hydrous minerals of the oxidized ore body into the anhydrous silicates and oxides of the present zinc deposit represents the next step in origin of the deposits. The ancient pre-Cambrian limestone has been deeply buried and intensely metamorphosed by heat and pressure since the oxidized ores near the old land surface were developed. All the minerals in the original rock have been recrystallized. Locally, they are coarsely crystalline aggregates. The extent off the metamorphism is only conjectural. The pressure was great enough to cause the limestone, or more correctly, the marble, to flow, distorting included rock masses and warping and folding the tabular ore body, and, as at Mine Hill, even completely shearing off a portion of the ore body itself. This movement was to the north and east according to Spurr and Lewis. The source of the heat necessary to produce the metamorphism may be two-fold: (1) that resulting from deep burial, and (2) that due to intrusion beneath, or nearby, of a batholithic mass, as postulated by Spurr and Lewis. The alteration of the oxidized zinc ores preceded the injection of the pegmatites (also a preCambrian event), which may have had their source in such a batholith but were injected long after the metamorphism of the ores. THE MINERAL ALTERATIONS INVOLVED The oxidized ore body containing the zinc, iron, and manganese is believed to have had the following mineral composition (in the order of abundance)
1. Hydrous iron-manganese oxides.
2. Hydrous zinc silicate.
3. Zinc carbonate.
4. Manganese carbonate. This order is based upon the relative abundance of the minerals in the present ore as given by Palache and furnished to him by the New Jersey Zinc Company. Palache's table follows.
MINERALOGICAL COMPOSITION OF THE FRANKLIN ORE
This ore body is believed to have been wholly within the limestone. The chemical changes necessary for the production of the present minerals are few and simple. They comprise dehydration and decarbonation changes (there is but scanty evidence of deoxidation) accompanied by a recrystallization of the ore body and country rock alike. Aside from the simple heat reactions of dehydration and decarbonation, the entire series of chemical reactions takes place among the three isomorphous elements: iron, manganese, and zinc. The microscopic studies of Ries and Bowen showed that there was a definite sequence in the recrystallization (regarded as crystallization by them, as they hold the ores are metasomatic replacements) of the ore minerals. That sequence is as follows:
As the table quoted from Palache's paper shows, franklinite is the most abundant mineral in the ores, willemite next, and zincite last. Palache does .not include tephroite in the list, but states that it is "an occasional rare accompaniment of willemite." It has been included in this analysis of the origin of the deposits because it is, even though rare, a constituent of the primary ores. Analyses of all the primary ore minerals are given in the tables that follow. When critically studied, these analyses reveal some rather astonishing facts concerning the presence of certain oxides, and the distribution of the essential elements: iron, zinc, and manganese, among them. The analyses are taken from Dana's "A System of Mineralogy," sixth edition. COMPOSITION OF FRANKLINITE (Dana. p. 227)
* Average of 3 only.
COMPOSITION OF WILLEMITE (Dana. p. 461)
COMPOSITION OF ZINCITE (Dana, p. 208)
COMPOSITION OF TEPHROITE (Dana, p. 458)
The composition of franklinite, as shown by these analyses, reveals the very significant fact that all the iron present is ferric iron, with the exception of one specimen. The analyses of five specimens do not show any ferrous iron. The formula for franklinite, if based upon these analyses, would be written somewhat differently. Yet the fact that ferrous oxide is isomorphous with the ZnO and MnO would make its presence theoretically possible in the mineral. The willemite also is essentially free from ferrous oxides, the average of eight analyses of the mineral from the Franklin area being only 0.66 per cent. The tephroite shows an average of 1.33 per cent ferrous iron in six specimens, but the very small percentage of this mineral present in the ores reduces this amount of ferrous iron to insignificant proportions. Zincite contains no ferrous iron, but has a little ferric oxide, doubtless included as an impurity. The absence of ferrous iron in all but one analysis of franklinite and the very small quantities of it in the other ore minerals is strong proof for the writer's theory of a fully oxidized ore body as the source of the present ores. Another feature of significance is the presence of MnO in all the minerals. The average content for each, and the number of analyses is given in the following table. AVERAGE PERCENTAGE OF MnO IN THE ORE MINERALS
Willemite and zincite are dominantly zinc minerals, but the franklinite contains an average of only 16.96 per cent and the tephroite 5.94 per cent ZnO. Thus we see that the minerals are dominantly zinc-manganese minerals. The only one containing significant amounts of iron is franklinite, and its iron is in the ferric state. The six analyses given of franklinite show an average of 63.6 per cent ferric oxide. Three of these analyses show Mn2O3 isomorphous with the ferric iron. Franklinite is the only mineral in the deposit that contains manganic oxide. Still another point of interest is that the tephroite of the district contains an average percentage of 9.06 MgO, larger than either the FeO or ZnO content. It has also an average content of 0.95 per cent of CaO making about 10 per cent of these two oxides. The formula for tephroite should be 2(Mn, Mg, Zn, Fe)OSiO2, if based on the material in the Franklin area. In order to show graphically the sequence of chemical changes taking place, the following chart has been made. The minerals in the oxidized ore body are on the left and arranged in the order of their probable abundance as already indicated. The resulting ore minerals are found on the right, but arranged in the order of their recrystallization as determined by Ries and Bowen. The dehydration of iron and manganese oxides and the zinc silicate would begin as soon as the temperature was raised, either by dynamic action or by igneous intrusion. According to Doelter,9 limonite may begin to lose its water at 50°C and is dehydrated at 150°C. Hemimorphite does not lose its water until heated red hot. Decarbonization of zinc silicate begins at a very low temperature, 90°C. according to Doelter. Manganese carbonate does not lose its CO2 until at a high temperature. Unfortunately, no determinations as to the temperature at which some of these minerals finally lose their H2O or CO2 are available. Some evidence bearing on the temperatures is shown in the burning of clays. The hydrous iron oxides lose their water early in the firing. Calcite (the chief gangue mineral of the ore) loses part of its CO2 at about 812° to 825° C. Such temperatures as these exceed the temperature of many magmas, and it seems improbable that the temperature of the Franklin limestone and its ores ever exceeded those given above. Furthermore, the reactions of the oxides with each other would certainly take place at lower temperatures and thus permit the carbon dioxide and water to go free.
The dehydration of the hemimorphite accompanied by decarbonization of, or reaction with, the MnCO3 would give rise to the willemite (the FeO in this mineral being derived from the MnCO3 as hemimorphite never shows any FeO). The tephroite was formed from the MnSiO3, of braunite, uniting with Zn2SiO4 and MnCO3, or possibly with ZnCO3, in a dolomite. The franklinite was formed by the dehydration of the limonite, manganite, and the slightly hydrous hematite and pyrolusite, in the presence of ZnCO3 and MnCO3. The one specimen (there may be others in the deposits) containing FeO was evidently formed from the reaction of an iron-rich zinc or manganese carbonate with the other oxides. Any excess of ZnCO3 became ZnO and was either manganiferous, originally, or reacted with some MnCO3 to become so. There is no reason to assume that these reactions took place rapidly, although they undoubtedly were all completed within one period that antedated the intrusion of the pegmatites. The pre-Cambrian period was long, and thus there was ample time for an infinite number of reactions to have taken place. The oxidized minerals involved are all commonly found in oxidized deposits, and many are found in the same association. They do not represent impossible combinations. It may be asked why braunite was assumed to have been present rather than rhodonite, and the answer is that rhodonite is more typically a mineral of certain vein deposits and is frequently found in schists. It could not be stated that it was not present, however, and if it was, it might well have been converted into tephroite. The reactions are dominantly temperature effects and attendant recrystallization. Deoxidation is not demanded. Two simple oxides: manganous and zinc, appear in every mineral, along with manganic and ferric oxides. If manganese dioxide (pyrolusite) was present in the oxidized ores, it would lose a part of its oxygen as the heat in the rocks increased and become Mn2O3. This is a simple laboratory method of preparing Mn2O3. The silicates which were formed merely acquired isomorphous elements made readily available by the decarbonation of the carbonates already intergrown with them and favored by the temperature. A more striking illustration of the part isomorphism may play in the formation of a series of minerals would be difficult to find. A comparison of the above changes with those suggested by Rastall will disclose several wide differences. Some of his suggestions are evidently impossible, as for instance, his reaction:
ZnCO3+Mn2O3=ZnO
Mn2O3+CO2 and 2ZnCO3+SiO2=Zn2SiO4+2CO2 Silica may have been present, but the evidence is against it. The franklinite of the district contains 63.60 per cent ferric oxide, indicating the presence in the original deposit of hydrous iron oxides. The theory of Ries and Bowen that the deposits are due to the replacement of limestone (the writer believes that the oxidized ores accomplished this) leaves one entirely in the dark as to the source of the solutions. These authors do not draw for us any concept as to the character of the solutions, doubtless because they recognized the difficulties involved. So far, no one has given us a concept as to the type of solution that could transport and deposit this unusual aggregate of minerals. The process outlined by the present writer does not call for any reactions out of the ordinary. THE LIMITS OF THE PRESENT DISCUSSION The writer has made no attempt to discuss the general geology or the details of the occurrence of the ore bodies. Neither has he discussed the pegmatites, or the development of the long list of rare minerals. He believes they have been fully and accurately described by the authors listed at the beginning of this paper. He does not subscribe to all the details as given by them, but does feel that the sequence of events is much as has been described by Spencer, Ries and Bowen, and Palache, and, to a certain extent, by Spurr and Lewis. The theory advanced here seeks only to trace the events that gave rise to the present zinc-manganese ore body before it was altered locally by subsequent intrusions. The writer believes that the mineralogy advanced here, and the reactions given, account, in a simple and reasonable manner, for the unique mineral aggregate of the present ore deposit. NOTES 1 Spencer, A. C., U. S. Geol. Survey, Franklin Furnace Folio, No. 161. Ries, H. and Bowes, W. C., Origin of the Zinc Ores of Sussex County, N. J., Econ. Geol., vol. 17, pp. 517-571,1922. Spurr, J. E. and Volney, J. Lewis, Ore Deposition at Franklin Furnace, New Jersey, Eng. and Min. Jour. Press, vol. 119, pp. 317-328, 1925. Palache, Charles, Paragenetic Classification of the Minerals of Franklin, New Jersey, Am. Mineralogist, vol. 14, pp. 1-18, 1929. 2 Rastall, R. H., The Geology of the Metalliferous Deposits, pp. 131, 305, 1923 3 Beyschlag, Vogt, and Krusch, Ore Deposits, vol. 2, p. 731, see also p. 735. 4 Buckley, E. R. and Buehler, H. A., The Geology of the Granby Area, Mo. Bur. of Geol. and Mines, vol. 4, 1905. 5 Emmons, S. F., Geology and Mining Industry of Leadville, Colo., U. S. Geol. Survey, Mon.12,1886. Emmons, S. F., Irving, J. D. and Laughlin, G. F., Geol. and Ore Deposits of the Leadville Mining District, Colo., U. S. Geol. Survey, Prof. Paper 148, 1927. 6 Blake, W. P., Zinc Ore Deposits near Hanover, New Mexico, Trans. Am. Inst. Min. Eng., vol. 24, pp. 187-195, 1894. 7 Rastall, R. H., ibid. p. 305. 8 Doelter, C., Handbuch der Mineralchemie, vol. 1, p. 444. 9 Doelter, C. ibid vol. 3 pt. 2 p. 714. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||