| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
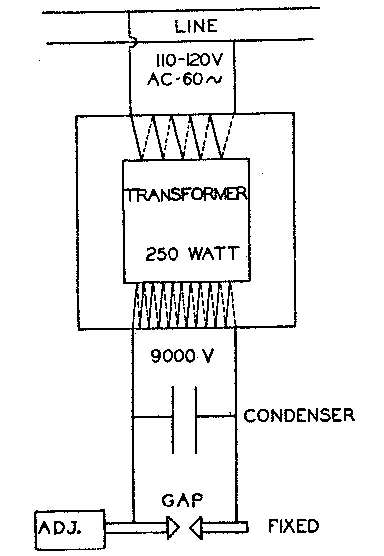
Volume 13, pages 330-333, 1928 THE PHOSPHORESCENCE AND FLUORESCENCE OF FRANKLIN MINERALS CHARLES PALACHE The use of ultraviolet light as yielded by the iron-arc spark-gap to determine the presence of willemite in mill-tailings has long been an important practice at Franklin. From it has grown up the constant use of this apparatus in determining the presence in the Franklin ores not only of willemite but also of a number of other minerals which give characteristic reactions to this form of stimulus. Since these reactions may not be generally known it seemed desirable to give a brief account of the apparatus used and of the observations which may be made with it. The spark-gap apparatus used in this laboratory is one made by the preparator, and modelled after the instrument manufactured for a short time by the General Electric Company and described by Mr. Andrews.1 Mr. Littlefield has prepared the following brief description and figure showing its design. Figure 1 shows the electrical layout of the apparatus which consists of three major parts: the transformer, the condenser and the gap. It was possible to obtain a suitable transformer from a radio supply-house. The condenser was made by placing thin sheets of copper in alternate layers with old photographic plates from which the gelatin had been removed. Eight sheets of copper 6"x6" were found to give the desired capacity. The gap was made with one adjustable and one fixed electrode, being enclosed in a cup-shaped receptacle of insulating material. The electrodes were provided with removable tips of iron about 3/16" in diameter. It is installed in a box having a holder for the gap on the outside and provided with a snap-switch for making connection with the line (lighting circuit). The apparatus differs from that described by Mr. Andrews only in refinement of construction. By the term fluorescence as here used is meant the color reaction given by some minerals when exposed to ultraviolet light. If this color endures after the light is extinguished the mineral is said to show phosphorescence. No observation of phosphorescence of very short duration is here included. The only Franklin mineral showing strong phosphorescence is willemite. It is well known that these phenomena are rarely given by pure materials but are caused by the presence of impurities of some sort generally in very small amounts. Hence these characters are variable in intensity and the same mineral may or may not react. Notwithstanding this variability, the characters are sufficiently well defined to be exceedingly useful both in determining the presence of a given mineral in the ore and in testing the purity of mineral separations where a fluorescent or phosphorescent mineral is involved.
Fig. 1. Electrical layout of spark-gap apparatus. In a recent article by L. J. Spencer2 attention is drawn to this property and the use of the mercury-vapor lamp as a source of, ultra-violet rays is described. The writer experimented briefly with the small "Labarc" used in this laboratory as a source of monochromatic light. Whether because it is a less powerful lamp than the one used by Dr. Spencer or because of unappropriate housing, the effects obtained were far less brilliant than with the iron-arc. It was particularly noted that fluorescence was less vivid but on the other hand several specimens showed phosphorescence after exposure to the mercury arc in which it had not been visible with the spark gap. It is of course quite to be expected that the range and intensities of ultra-violet waves in the two light sources would be so different as to produce varying effects. WILLEMITE. Generally fluorescent and sometimes strongly phosphorescent with a green color. The normal Franklin willemite shows both characters. The purest white willemite, however, gives a weak or no reaction. Troostite, the variety of willemite high in manganese, also gives a weak or negative reaction. The veins of radiated fibrous willemite which contain but a little manganese have a phosphorescence so strong that the vivid green glow lasts five minutes or more after brief exposure to the iron-arc. The so-called "black" willemite which is a very pure willemite crowded with minute inclusions of franklinite does not fluoresce. If no spark-gap is available the phosphorescence of willemite can be very well exhibited by burning a short length of magnesium ribbon close to the specimens. In a dark room the phosphorescent glow is strong. CALCITE. Pure calcite is negative. Most of the calcite and dolomite of the Franklin ore is manganiferous and then fluoresces in various tones of vivid red to pale pink. In some cases what is apparently calcite has a faint violet fluorescence but the nature of the impurity here is unknown. CALCIUM-LARSENITE. This new calcium lead silicate has a very vivid fluorescence of lemon yellow color. It is more intense than the fluorescence of willemite. Unlike the latter it does not phosphoresce. LARSENITE is negative or pale violet. PECTOLITE. Gives a fluorescence of pure yellow tone probably due to its slight manganese content. CLINOHEDRITE. Fluoresces with a slightly orange yellow tone not so vivid as pectolite or calcite. It is not easy to distinguish from pectolite by the fluorescence. MARGAROSANITE. Fluoresces with a rather lively pale violet color. HARDYSTONITE. Fluoresces with a dull faint violet tint, sometimes negative. ROEBLINGITE. Is negative or gives a very pale pink fluorescence. HEDYPHANE. A bluish gray fluorescence, not very distinct. None of the many other minerals at Franklin of a composition more or less related to the above show fluorescence. The test is, therefore, an effective one for selecting the comparatively small number of species which react. The description of these fluorescent colors in ordinary color terminology is apt to be misleading. No one who has not seen it can realize the peculiar quality of the color in a strongly fluorescent mineral. Its whole mass seems to glow more like a molten metal than a solid. The appearance of a specimen of calcium-larsenite for example is astonishing. Seen in daylight it is colored throughout white or grayish except for the interspersed grains of black franklinite. But under the spark-gap it is a medley of color. The pale yellow fluorescence of the calcium-larsenite and the green of willemite dominate the color scheme. In cavities, clinohedrite shows areas of orange yellow and larsenite, a very pale violet tint. Hardystonite, too, is violet toned while scattered grains of calcite glow with a vivid red. NOTES 1 General Electric Review, Vol. XIX, No. 4, p. 317, 1916. 2 Fluorescence of willemite and some other zinc minerals in ultra-violet rays. Mineralog. Mag., 21, 394, 1927.
|