| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
Volume 27, pages 281-300, 1942 ESPER S. LARSEN, 3d, Harvard University, Cambridge, Mass.* ABSTRACT The Fairfield variscite deposit is located about 50 miles south of Salt Lake City. The variscite occurs as nodules in a highly brecciated zone in limestone. The nodules are veined and surrounded by several other calcium aluminum phosphates; other, rarer phosphate minerals are in cavities in the nodules. Miscellaneous additional data are given on some of the minerals. Weissenberg x-ray studies were made on wardite, deltaite, and gordonite. On the basis of x-ray powder photographs, wardite is shown to be related structurally and chemically to millisite, and deltaite to pseudowavellite. The paragenetic relations of the minerals are described in detail. Variscite was deposited as nodules, and the other phosphate minerals are alteration and replacement products of the original variscite. The alteration history can be divided into four stages: In the first stage, quantitatively the most important, the bulk of the pseudowavellite was deposited with some deltaite, then millisite and wardite with some deltaite and lehiite, and finally more pseudowavellite and deltaite. The second stage comprises the crystal-forming minerals in cavities of variscite and pseudowavellite; these are, in their probable sequence, gordonite, englishite, montgomeryite, overite, and sterrettite. The third stage represents the deposition of a small amount of pseudowavellite, and the fourth, the deposition of the members of the apatite group. Each of these stages has characteristic chemical qualities. The variscite was formed by phosphatic groundwaters descending along open fissures; the source of the phosphate was probably a phosphorite bed, weathering at the surface. The deposition of the variscite was probably caused by the reaction of aluminous material with the phosphatic waters. Later groundwater, no longer phosphatic, reacted with the variscite to form the alteration minerals. The different chemical characteristics of each group of alteration minerals reflect the changing composition of the groundwater which in turn was due to the weathering of varying surface materials. TABLE OF CONTENTS, PART 1.
Introduction ......................................... 282 INTRODUCTION The descriptive mineralogy of the Fairfield variscite deposit has been known since the publication of Larsen and Shannon's paper (1930b) on the subject, but little has been written about the paragenetic relations of this or any other variscite deposit. Since the mineral assemblage at Fairfield is unique, so far as known, there is the logical assumption that the conditions of origin were perhaps also unique. An investigation of specimens from the deposit has been made with this in mind. This study was undertaken at the suggestion of Professor Charles Palache, who had acquired a large new collection of Fairfield specimens for the Harvard Mineralogical Museum from Messrs. Arthur Montgomery and Edwin Over. The writer was fortunate in having this collection at his disposal. Moreover, Mr. Montgomery very generously furnished a large amount of additional material whenever it was needed. The Fairfield variscite deposit has been known to mineral collectors for a number of years. The variscite is present as brilliant green nodules, which are veined and surrounded by bands of yellow, white, and gray alteration minerals. The nodules are found in a shattered and altered limestone (the "Great Blue" of Upper Mississippian age). The deposit is located about 50 miles south of Salt Lake City in the southern end of the Oquirrh Range; more exactly, on the north slopes of Clay Canyon, five miles west of the town of Fairfield. It can be reached by a poor road from Fairfield. The nodules were first discovered in 1893, and they were mentioned by Kunz (1895) in the Mineral Resources bulletin as a new type of occurrence for variscite. Other similar variscite deposits in Utah and Nevada have since been found. The Fairfield deposit was mined at the surface in a small way for a number of years, producing a considerable quantity of variscite suitable for semi-precious gems and ornamental stone. For the past twenty years or more the deposit has lain idle with the exception of sporadic mineral collecting. In 1937 the deposit was claimed by Messrs. Montgomery and Over, who spent several months in 1937 and 1939 doing the first successful underground work. Their aim was to collect mineral specimens rather than gem material. The specimens used in this study comprised part of the material collected by them. LITERATURE The literature on the Fairfield deposit is very meagre. Kunz (1895) gave a very brief description of the deposit, and suggested the name "utahlite" for the gem variscite. Packard (1894) analyzed some of the material from one nodule and identified the green material as variscite. Wardite from Fairfield was described by Dennison in 1896. Sterrett (1905, '06, '07, '08, '09, '10) briefly described the deposit and the types of gem material produced. In 1930, Larsen and Shannon published the first detailed description of the mineralogy of the nodules, and recorded eight new minerals and the general occurrence of the minerals within the nodules. Pough has recently described the morphology of wardite (1937 a) and gordonite (1937 b). McConnell (1938) discussed lewistonite and dehrnite from Fairfield in their structural relation to other members of the apatite group. Gilluly (1932) described the geology of the Fairfield quadrangle but did not describe the variscite deposit. PURPOSE AND METHODS OF THE STUDY This investigation was undertaken, in part, to expand the known mineralogy of the deposit, and in part to consider the origin of the variscite and its alteration products. All the minerals were studied optically, and where suitable single crystals were available, their structural lattice was determined by the Weissenberg x-ray method. X-ray powder photographs were made of all the minerals observed and were used for purposes of identification and comparison. Thin sections were used to study some of the textural features. The spatial relations and the sequence of the minerals were studied more fruitfully in broken nodules under the binocular microscope. The work was carried out entirely in the laboratory; the field descriptions have been taken from the literature and from personal communications from Mr. Arthur Montgomery. The writer believes that further field investigation of this and other variscite deposits would be of value in testing the validity of some of the suggestions proposed in this paper. ACKNOWLEDGMENTS The principal collection of nodules used in this study was furnished by the Department of Mineralogy and Petrography of Harvard University, where the work was done. They also financed the chemical analyses and the making of thin sections Professor Charles Palache suggested the study and offered numerous valuable suggestions. Professor Esper S. Larsen gave valuable advice in the course of the work, and very kindly read and criticized the manuscript. Professor C. S. Hurlbut, likewise, read and criticized the manuscript. Drs. Wallace Richmond, Clifford Frondel, and C. W. Wolfe assisted greatly in the x-ray work. Dr. William T. Pecora contributed through many discussions. I wish especially to thank Professor Harry Berman for his very constant interest in the work and his many criticisms, suggestions, and correction, both in the course of the investigation and in the preparation of the manuscript. Mr. Arthur Montgomery furnished unstintingly an abundance of material used throughout the study. GENERAL GEOLOGY Gilluly (1952) has described in detail the geology of the Fairfield quadrangle; the following brief description of the general geology of the area is taken from his publication and is summarized below. The Fairfield quadrangle includes the southern end of the Oquirrh Mountains, the eastern extremity of the basin and Range province. The rocks, dominantly Paleozoic sediments, are in a series of north - north-west trending open anticlines of variable dip. The stratigraphy is described in detail by Gilluly. The aggregate thickness of the Paleozoic strata is near 25,000 feet, dominantly limestones. These strata were compressed into the present anticlines at the end of the Paleozoic or early in the Tertiary. Following this, and in the Tertiary, there were extruded several thousand feet of latitic volcanics now found principally to the north; the same magma later rose toward the surface forming stocks, dikes, plugs, etc., with which the ore deposits of the area are associated. Considerable local faulting is attributed to the intrusions.. Erosion during the Oligocene and early Miocene developed a "subdued mature surface" where the present mountains are located. From late Miocene or early Miocene to the present, the Basin and Range faulting has tilted the Paleozoic strata to their present positions, which, together with accompanying erosion, has developed the present mountainous surface. Huge alluvial deposits have formed in the present valleys. GEOLOGY OF THE DEPOSIT The variscite deposit lies on the east flank of the Ophir Anticline, the southernmost extension of the Oquirrh Mountains. It is located entirely in one sedimentary formation, the "Great Blue" limestone of upper Mississippian age. Gilluly (1937, p. 29) has described this formation as follows: "The 'Great Blue' limestone consists of a lower and an upper limestone, separated by shaly beds herein named the Long Trail shale member. The lower limestone member, between the top of the Humbug and the base of the Long Trail shale, is . . . about 500 feet thick . . . (The Long Trail member) is about 85 feet thick. MINERALOGY OF VARISCITE NODULES "Overlying the Long Trail shale is the upper limestone member of the 'Great Blue' limestone, consisting of blue-gray limestone like that beneath the shale and containing sporadic chert layers, some sandy limestone, and a very subordinate quantity of black shale . . . 2750 feet (is) the most probable thickness of the upper limestone member .... "Other than the Long Trail shale member, no good marker beds were discovered in the 'Great Blue' limestone; it is a monotonous thick series of limestones throughout. In local areas, as at Mercur, individual thinner beds, mostly shales, were recognized in the formation...." The variscite occurs above the Long Trail shale member, but how far above is not known. The limestones have erratic dips and strikes in the neighborhood of the deposit, and at the deposit strike about N. 50° W. and dip 22° N. (Sterrett, 1908, p. 856). No igneous rocks were mapped near Clay Canyon. Two miles northwest is a large rhyolitic plug (Eagle Hill) which may be genetically associated with the ore deposits near Mercur, farther to the north. There is no recurrence of intrusives south of Eagle Hill. The structure of the deposit itself is only briefly recounted in the literature, and has been known only from surface workings. Kunz (1895, p. 602) wrote: "The rock is a crystalline limestone, with layers of black pyritiferous slate. In the latter occur the nodules, varying from the size of a walnut to that of a cocoanut . . . . " Sterrett (1908, p. 856) visited the deposit after the surface opening had been considerably developed, and wrote: " . . . Development consists of a tunnel 110 feet long driven nearly north into the hill, and an open cut with a small incline. The tunnel did not cut the variscite lead. The country rock exposed in the workings is a black limestone, which strikes about N. 50° W., with a dip of 22° N. The variscite lead has a steeper dip to the north, nearly 45°, with approximately the same strike as the limestone. The variscite occurs in concretionary nodules in a brecciated, more or less decomposed, zone. Practically everything in this zone has a nodular shape, including the blocks of limestone breccia, etc. Chert forms a prominent part of the filling of the mineralized zone and has been fractured and cemented by calcite seams and limonite stains. The nodules of variscite range from one-fourth of an inch to over 4 inches in thickness. The nodules have been more or less fractured, and the cracks have been filled in with yellow and white phosphate minerals. Some of the larger nodules contain two or more smaller nodules or irregular masses of variscite, inclosed in yellow and white matrix or shells. Most of the nodules are surrounded by banded layers of the yellow phosphate and some have white coatings also." Messrs. Montgomery and Over have recently done successful underground work on the deposit, and very kindly furnished data for the following more detailed description. Their work consisted of reopening a horizontal tunnel running into the hillside below the outcrop but which missed the mineralized zone (Sterrett, 1908). Lateral drifts from this tunnel struck the variscite area, and the zone of mineralization was followed from the lateral drift, to the surface, a distance of about 100 feet. The mineralized phosphate zone dips about 25° to the east, slightly steeper than the limestone, and the pitch of the zone is more northward than the true direction of dip of the limestone. The mineralized zone is one of extreme fracturing and brecciation, with several small through-going vertical faults, and small dip faults. The nodules are centered about vertical fractures, and generally occur along bedding fractures near the main vertical fractures. There are generally two, and locally three, well-defined phosphate streaks. These are discontinuous, and have a maximum vertical range of eight feet, and a lateral extent of about three feet. Where the mineralization is strongest, two or three main streaks may be close together, but separated by barren limestone, the whole having a width of ten feet or less. A few isolated patches or stringers of nodules were found away from the main fractures, but in diminished quantities. The nodules are found in masses of apparently altered limestone of which most is silicified or limonitized; as patches or layers parallel to the bedding surrounded by iron-stained earthy material; as angular fragments in brecciated limestone (silicified?) - and as small nodules enclosed in angular alunite fragments. Many of the nodules are fractured and faulted, and a number of the bedding layers of nodules are offset, probably by late fracturing and small movements along them. The limestone in the mineralized area, and to some unknown distance away from it, is almost entirely silicified. Large cherty-appearing nodules and angular fragments of alunite are common. Late quartz and calcite were found in some of the fractures. The amount of mineralization is considerably greater in the lower part of the workings, where the mineralized zone appears to expand. The nodular material at depth is completely altered to yellow pseudowavellite and a white mineral which may be pseudowavellite. DESCRIPTIVE MINERALOGY The minerals occurring in or associated with the nodules are discussed here from a strictly descriptive point of view; the details of their occurrence and sequence are considered in a later section. Each mineral found is described briefly; where new data have been gathered, they are given in detail. Morphological, structural, and chemical studies were made on suitable material if the data had not appeared in the literature. Three new phosphate minerals, overite, montgomeryite, and sterrettite, were found in the course of the study, and full descriptions of them are given elsewhere (Larsen, 1940; Larsen and Montgomery, 1940). In all, fourteen species of phosphate minerals have been determined in the deposit; eleven were first described from this locality, and most of them are peculiar to it. Following the scheme of classification of the phosphate minerals of Strum and Schroeter (1939), the minerals in the Fairfield deposit fall in the following groups:
Group 1. Anhydrous phosphates without additional anions.
Group 2. Anhydrous phosphates with additional anions.
Group 3. Hydrous phosphates without additional anions.
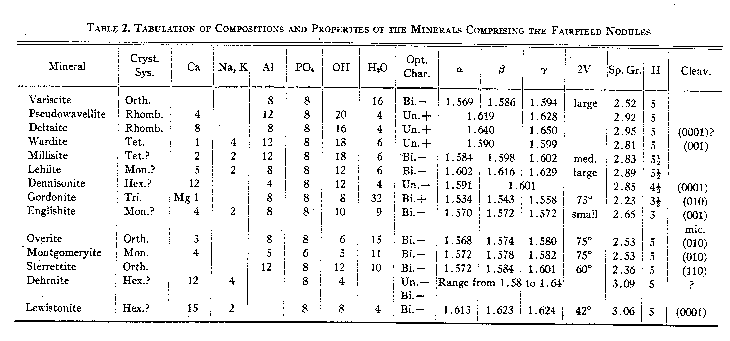
Group 4. Hydrous phosphates with additional anions. Overite was placed in Group 3 by Strunz and Schroeter on the basis of the tentative but incorrect formula Ca3Al6(PO4)8·20H2O, published by Larsen (1939). Table 2 presents the chief properties of all the phosphate minerals found in the nodules. VARISCITE - AlPO4·2H2O - orthorhombic The identity of variscite has until recently been confused in the literature. Breithaupt (1830) described the mineral peganite and in 1837 described variscite. Moschetti (1917) and Schaller (1925) have shown that the two minerals are identical and that the original analyzed peganite probably contained some wavellite. The callainite of Dana may be variscite (Damour, 1865), although McConnell (1940) has lately questioned . this. Lucinite, described by Schaller (1916) has been shown by Larsen and Schaller (1925) to be identical with variscite. Moreover, the euhedral material from Lucin, Utah, described by Schaller (1912 a & b) as variscite, has been shown by Larsen and Schaller (1925) not to be variscite but a new dimorphous form of AlPO4·2H2O, named by them metavariscite; this error has not been fully recognized by Hintze (1931) in his description of the crystallography of variscite. The names utahlite, chlorutahlite, and amatrice have been used as trade names for gem variscite from Utah. All these names therefore represent but two species, variscite and metavariscite. Variscite is isostructural with strengite (Schaller, 1912 a & b) and recent studies (Strunz and Sztrókay, 1939; McConnell, 1939, 1940) have shown it to belong to the isodimorphous group including metavariscite, phosphosiderite, strengite, and others. The variscite of the Fairfield deposit is a fine grained, massive, green aggregate, invariably in nodular form or as residuals in replaced or brecciated nodules. These nodules are the finest ever found and when polished make very handsome specimens. The color varies from a vivid emerald green to a lighter pea green. The colors are generally mottled over large areas, and the outer edges of the variscite masses in some nodules have a narrow line of darker green. Dark green lines of variscite, cutting massive variscite, can be seen on polished surfaces; this is probably a recrystallized variscite which has "healed" small incipient fractures. A very minor amount of variscite is scattered through white powdery layers of a mineral related to pseudowavellite; Larsen and Shannon (1930 b) found this material to be in tiny crystals, coarser than the massive green mineral; the material studied by this writer was very fine grained and no crystals were found. Some of the white powdery layers have a faint greenish tinge on the surface facing the massive variscite, due to a concentration of loose variscite grains. No crystals of variscite have been found at Fairfield, although the similar deposit at Lucin has yielded crystals (Schaller, 1915; Larsen and Schaller, 1925). Two analyses of the Fairfield variscite have been published (Packard, 1894; Schaller, 1916); they both indicate a hydrous aluminum phosphate with some iron replacing aluminum. By analogy with strengite, the unit cell content of variscite is 2(AlPO4·2H2O) (Strunz and Sztrókay, 1939; McConnell, 1940). PSEUDOWAVELLITE - CaAl3(PO4)2(OH)5·H2O - rhombohedral DELTAITE - Ca2Al2(PO4)2(OH)4·H2O - rhombohedral Pseudowavellite and deltaite are shown here to be isostructural and possibly isomorphous; for this reason they are discussed together. Pseudowavellite was described by Laubmann (1922), and was identified as the dominant alteration mineral of the variscite at Fairfield by Larsen and Shannon (1930). It has been described from few localities, but there is little doubt that the mineral comprising the yellow crusts associated with variscite at the Utah and Nevada variscite deposits (see Sterrett, 1905, '06, '07, '08, '09, '10) is identical with the Fairfield pseudowavellite; specimens from Amatrice Hill, seen by the writer, contain pseudowavellite optically indistinguishable from the Fairfield material. Deltaite was originally described from Fairfield by Larsen and Shannon (1930 b), and so far as is known to the writer has been found at no other locality. Three types of occurrence of pseudowavellite were described by Larsen and Shannon. The most prominent mineral of the nodules is a yellow pseudowavellite forming successive layers on the outer parts of the nodules; this occurs as very fine fibers, either subparallel or matted, and having average indices of refraction ω=1.618, ε =1.623. Vitreous crusts and veinlets, and spherules, ranging in color from yellow to gray, are made up chiefly of granular pseudowavellite (ω=1.622, ε =1.631) with some deltaite. White chalky crusts surrounding variscite are composed principally of pseudowavellite with indices ω=1.619, ε =1.627; this is very fine grained, in part either isotropic or so fine grained, as to appear so. Analyses of these three types are given by Larsen and Shannon (1930 b, Tables 2 and 3). The same writers likewise describe three types of deltaite. As noted above, deltaite occurs intergrown with some vitreous pseudowavellite; this deltaite has indices ω=1.641, ε =1.650, and in general has roughly euhedral outlines, apparently that of a trigonal prism, in very minute crystals. Gray cherty-looking crusts near variscite are made up of matted fine fibers of deltaite with indices ω=1.630, ε =1.640. White chalky layers between the gray deltaite and the variscite kernels are considered to be deltaite by Larsen and Shannon; it is very similar optically to the white chalky pseudowavellite, and has indices ω=1.621, ε =1.629, but is chemically somewhat different. This material was not seen by the writer, or else has been confused with the white chalky pseudowavellite. These three types of deltaite have been analyzed by Larsen and Shannon (1930 b, Table 4). Three other types of deltaite have been seen by this writer. On one micromount are colorless minute crystals, apparently rhombohedra with a base, whose indices of refraction are ω=1.640, ε =1.650. Very similar material, but canary yellow in color, occurs as sugary aggregates of very tiny crystals lining cavities in the nodules, chiefly in lenticular openings between pseudowavellite shells. These yellow crystals have a maximum dimension of 0.02 mm. and thus are far too small to study with the goniometer. Upon immersion under the microscope they are seen to be almost cubic in outline, but extinguish parallel to the face diagonals, suggesting that they are rhombohedra; rarely a corner is truncated by what is probably the base. This material has indices ω=1.641, ε =1.651. A third type of deltaite comprises lavender colored trigonal prisms and massive aggregates of lavender to pale blue bands in a few of the nodules. This material has indices essentially identical with the types noted above. The lavender crystals constitute the only deltaite suitable for crystallographic and x-ray Weissenberg study; the results of this study are given below. X-ray powder photographs of the principal types of pseudowavellite and deltaite were taken, and they are all indistinguishable. It is assumed therefore that the minerals, including all their various types, are isostructural, and since the lattice spacings indicated by the photographs all appear to be very similar, it is assumed that the lattice dimensions of all the types are essentially identical. Lavender crystals of deltaite A few small nodules contain small lavender crystals of deltaite lining cavities in pseudowavellite. These crystals occur as single units and sub-parallel aggregates growing with the c-axis normal to the cavity walls. The aggregates are intermixed with fine aggregates of pseudowavellite. Radial groups of a mineral belonging to the apatite group perch upon crystals in the cavities. The lavender deltaite is uniaxial positive, with the indices of refraction: ω=1.640, ε =1.651. It has been impossible to prepare for chemical analysis a sample of the deltaite even moderately free from admixed pseudowavellite, since the densities of the two minerals are practically equal, preventing gravity separation, and the fineness of grain prohibits hand sorting.
The crystals of lavender deltaite are all simple, consisting of an elongated
trigonal prism, terminated by the base. Their maximum dimension is near 0.2 mm.
The base c{0001} reflects brilliantly, and on some of the crystals shows minute
steps or striations parallel to the prism. The prism is rough with deeply
grooved faces giving practically no reflections on the goniometer; a very weak
train of reflections extends from the prism toward the base on some crystals.
The prism, to fit the orientation chosen for the unit cell, is a{10
The character and orientation of the unit cell were determined by the
Weissenberg x-ray method. Rotation, and zero, first, and second layer
Weissenberg photographs about [0001], and rotation and zero layer Weissenberg
photographs about [10
The space group is C3v5-R3m as shown by the following observed reflections:
For hkil, h-k+1=3n The rhombohedral cell agrees with the apparent rhombohedral form of the colorless and yellow crystals of deltaite described above. Chemical relationships Using the rhombohedral unit cell volume derived from the lavender crystals of deltaite, and the analysis of optically similar deltaite published by Larsen and Shannon (1930 b, Table 4, column 1), the calculated cell content of deltaite is close to: Ca2A12(PO4)2(OH)4·H2O. Other available analyses of deltaite (Larsen and Shannon, 1930 b, Table 4) show an excess of Al and a deficiency of Ca over that required for the above formula. Since x-ray powder photographs of deltaite and pseudowavellite show the two minerals to be isostructural and to have essentially identical cell dimensions, the rhombohedral cell volume determined for deltaite was used in calculating the unit cell content of pseudowavellite from the analyses given by Larsen and Shannon (1930 b, Tables 2 and 3). The cell content thus calculated closely approximates: CaAl3(PO4)2(OH)5·H2O. with an excess of Ca and a deficiency of Al in some. The analyses of deltaite show a moderate deviation in composition from that expressed in the formula toward that of pseudowavellite, and the analyses of pseudowavellite show a similar deviation toward deltaite. The two minerals therefore seem to constitute an isomorphous series, at least partly miscible, in which one atom of Ca in deltaite can be exchanged for one atom of Al, with a consequent adjustment of the (OH) content to balance the valence change, to form pseudowavellite. The formula for the series can be written: Ca(Ca,Al)Al2(PO4)2(OH)4-5·H2O. The formulae for the two minerals given by Larsen and Shannon do not suggest the isostructural character of the two minerals, or their apparent isomorphism.
WARDITE - CaNa4Al12(PO4)8(OH)18·6H2O - Tetragonal Wardite was originally described by Dennison (1896) from Fairfield, Utah. Lacroix (1910) described the mineral soumansite from Montebras, France, and this was shown by Larsen and Shannon (1930 b) to be identical with wardite. Larsen and Shannon analyzed the wardite from Fairfield and determined its physical and optical properties. Pough (1937 a) has described the morphology of the Fairfield wardite. Montebras is the only occurrence of wardite mentioned in the literature outside of Utah; Zalinski (1909) and Pepperberg (1911) mention it as a probable constituent of the Amatrice Hill and Lucin variscite deposits. Wardite at Fairfield occurs as subparallel aggregates of coarse fibers and aggregates and crusts of crystals, some individual grains exceeding one millimeter in diameter. They are most commonly blue-green, but grade to colorless. The crystals are tetragonal and characteristically pyramidal, terminated by the base. Most of the material is uniaxial positive, but some crystals are divided into four biaxial segments with a small 2V; this has been described by Lacroix (1910). X-ray powder photographs of wardite and millisite are similar, indicating a close structural relationship; this is discussed in the section on millisite. Rotation, and zero and first layer Weissenberg photographs about [001] and rotation and zero layer Weissenberg photographs about [110] were taken. The mineral proved to be tetragonal, as shown by Pough from the morphology, and has the following unit cell dimensions: ao=7.04 Å, co =18.88 Å, both ±0.02 Å The space group is C42-P41, or C44-P43 as shown by the observed reflections:
for h00, h= 2n hk0, h0l, hkl - all present The choice of axes agrees with that of Pough. The ratio of ao:co= 1: 2.682 indicates that Pough's choice of the c-unit is half the true unit; using Pough's morphological values, a: c=1: 2.6234. These two values differ by three per cent, which is not surprising considering the poor quality of the crystals and the great range of Pough's measured values. The ratio derived by x-ray methods is probably better, and has been used in calculating the angle table (Table 1) for the forms observed by Pouch. Included in the table are the measured mean values from Pough (1937a, Table 1). The form symbols have been changed to conform with the structurally determined units; the transformation is: Pough to Larsen 100/010/002. The dominant form t, given the symbol { 13.0.12 } by Pough, would become {13.0.24} with the present choice of unit, which is even less likely as a dominant form than with Pough's symbol. It seems most probable that this form should have the simple symbol t (102 } ; the p value of {102} by the new units is 53°17', which is lower by twelve minutes than any of the measured p values given by Pough, but his measured range is greater than four degrees, indicating both poor and inconsistent faces. Form {907} of Pough seems more probably to be {203} in the new setting ( {403} in the old); it is given as a doubtful form at best. TABLE 1. WARDITE-ANGLE TABLE (Calculated from x-ray data) Tetragonal pyramidal?-P41-3 po=2.6818, a:c=1:2.6818
The atomic content of the unit cell was calculated from the analysis of wardite published by Larsen and Shannon (1930 b, Table 1), and their value of the specific gravity 2.81, which new measurements by the writer checked closely. The unit cell contains CaNa4Al12(PO4)8(OH)18·6H2O. This differs from the formula for wardite given by Larsen and Shannon in having two less molecules of H 2O. The specific gravity calculated for this formula and the unit cell volume given above is 2.87, which compares poorly with the measured value 2.81.MILLISITE - Ca2(Na,K)2Al12(PO4)8(OH)18·6H2O - Tetragonal? Millisite was described from Fairfield by Larsen and Shannon (1930 b), and this is the only known locality for the mineral. Millisite is invariably associated with wardite as alternating layers in spherules or crusts. It is light gray to white in color, and normally is present as layers of fine fibers normal to the layering. No crystals of the mineral have been found. Where it coats crystals of wardite, it is not in crystallographic continuity, but is present as a thin shell with a finegrained aggregate structure. Material sufficiently coarse indicates that it is biaxial positive with a moderate 2V, and indices of refraction slightly lower than wardite; fibers show negative elongation. Millisite and wardite are very closely related chemically, as shown by the formulae:
Millisite Ca2(Na,K)2Al12(PO4)8(OH)18·6H2O in which potassium is very subordinate in wardite and is present in the approximate atomic ratio Na:K=2:1 in millisite. X-ray powder photographs of the two minerals are similar and the principal spacings are nearly identical; thus the unit cell volume of wardite ( Vo= 936 cu. Å) was used to calculate the probable unit cell content of millisite. The above formula indicates the probable cell content, assuming the measured specific gravity 2.83 from Larsen and Shannon. The calculated specific gravity for this formula is 2.87. In all cases where the two minerals occur together (and they usually do) they are separate and distinct without any gradation from one to the other, and each maintains its separate properties. For this reason they are to be considered separate species and not necessarily members of a series. LEHIITE - Ca5Na2Al3(PO4)8(OH)12·6H2O Lehiite was described from Fairfield by Larsen and Shannon (1930 b) and this is the only known occurrence of the mineral. Material identified by this writer as lehiite differs somewhat from that described by Larsen and Shannon. It forms dense, light gray layers on the outer shells of the nodules, and is made up of fine to moderately coarse fibers generally in subparallel bands. It contains a few thin bands of coarse wardite. The original description of lehiite gives the optical properties: α =1.600, β =1.615, γ =1.629, 2V large, optically negative, and a large extinction angle. The material seen by this writer was very finely fibrous with approximately parallel extinction; the fibers have negative elongation and the indices 1.605 parallel to the length, 1.620 normal to the length. It is too fine grained to determine its optical character. Although its optical properties do not coincide with those in the original description, its appearance and general range of indices of refraction suggest that it is lehiite. An x-ray powder photograph of this lehiite shows it to be unrelated to any of the other minerals found in the nodules. It is apparently not related chemically to any other known mineral. DENNISONITE - Ca3Al(PO4)2(OH)3·H2O Dennisonite, described from Fairfield by Larsen and Shannon (1930 b), was not found in the material studied by the present writer. The chemical formula suggests a relation to the pseudowavellite-deltaite series, such that one atom of Al in deltaite is replaced by one atom of Ca to form dennisonite; however, its optical properties are completely unrelated in any serial fashion with those of pseudowavellite and deltaite. It is said to occur in cavities in pseudowavellite and deltaite as fibrous, white botryoidal to spherulitic crusts. GORDONITE - MgAl2(PO4)2(OH)2·8H2O - Triclinic Gordonite from Fairfield was described by Larsen and Shannon (1930 b) and related by them to paravauxite on the basis of chemistry and similarity in the interfacial angles of a few poor crystals. Pough (1937 b) described in detail the morphology and showed the morphological relation to paravauxite, the ferrous equivalent of gordonite. It occurs most abundantly as prismatic triclinic crystals in bands of subparallel aggregates on or near variscite. It is most commonly smoky white to colorless; rare crystals are pale pink at their tips, or are pale green. Its prominent {010} cleavage and crystal form serve to distinguish it readily from the other minerals occurring with it. The lattice constants of the unit cell were determined by x-ray methods. Rotation and zero layer Weissenberg photographs were taken about the three axes chosen by Pough, and a first layer Weissenberg was taken about [010]. Pough's setting and choice of units agree with the normal setting indicated by the x-ray study. The following are the lattice constants:
The crystals are apparently holohedral, so the probable space group is P
The form S { Measured Range Best Average Calculated φ ρ φ ρ φ ρ -84°51' -87°16' 65°13 69°40' -86°21' 68°04' -86°02½' 68°06' The atomic content of the unit cell was calculated from the analysis of gordonite given by Larsen and Shannon (1930 b, p. 333), using the value of Vo given above. and the specific: gravity 2.23. The specific gravity was determined from seven small samples (2½ to 7 mg.) of clear crystals on a microbalance; the average of these measurements was 2.23, as compared to Larsen and Shannon's value 2.28. The unit cell content is expressed by the formula given by Larsen and Shannon: MgAl2(PO4)2(OH)2·8H2O The calculated specific gravity of this is 2.22 for the above value of Vo. Englishite - Ca4K2Al8(PO4)8(OH)18·9H2O - Monoclinic? Englishite was described from Fairfield by Larsen and Shannon (1930 b) and has been noted from no other locality. It occurs as subparallel aggregates of flexible plates, and has a white pearly luster on its very perfect micaceous cleavage. It is found in cavities with wardite, and replacing variscite. A Laue photograph was taken of a cleavage flake with the x-ray beam normal to the cleavage surface The photograph was poor, due to the bent and aggregate nature of the cleavage flake, but showed a single plane of symmetry, indicating that the mineral is monoclinic. If the cleavage is considered the base, the optical orientation is: Z=b Y near a, Z near c.
OVERITE - Ca3Al8(PO4)8(OH)6·15H2O
-Orthorhombic These three new minerals were discovered in the course of this study and have been described recently in detail (Larsen, 1940; Larsen and Montgomery, 1940). Their chief properties are summarized in Table 2. APATITE GROUP Larsen and Shannon (pp. 324-327) described the two minerals, dehrnite and lewistonite, from the Fairfield nodules, and these have been shown by McConnell (1938) to belong to the apatite group. These minerals as described show considerable variation in their optical properties, and frequently have cores which are sharply distinct optically from the outer parts of crystals. They are found as hexagonal prisms, and subparallel aggregates of prisms; the crystals are commonly divided into six biaxial segments. In one type of dehrnite, the crystals show a uniaxial core surrounded by six biaxial segments; the core has the indices of refraction: ω=1.640, ε =1.633, and the border: ω=1.585, γ = 1.600. Another type, considered to be dehrnite by Larsen and Shannon, occur, as botryoidal crusts in cavities in pseudowavellite and deltaite; these are made up of coarsely crystalline units, some divided into six segments, and are biaxial negative with a small 2V, and the indices of refraction ω =1.610, β =1.619, γ = 1.620. Lewistonite likewise shows wide variation in its properties. Stout hexagonal crystals associated with oolites of pseudowavellite are divided into six biaxial negative segments, with 2V = 42°, and α = 1.613, β=1.623, γ=1.624. Some of these have uniaxial cores with ε near 1.60. An amygdule is made up of stout fibers, apparently uniaxial negative, and ω=1.621, ε =1.611. Larsen and Shannon give the formula for dehrnite : 14CaO·2(Na,K) 2O·4P2O5·3(H2O,CO2) and for lewistonite: 15CaO·(K,Na)2O·4P2O5·8H2O. Lewistonite contains less alkalies and considerably more water than dehrnite. Their optical properties overlap considerably so that it is impossible to distinguish between them.Other members of the apatite group, as shown by x-ray powder photographs, are present in the nodules. These have not been studied chemically; optically ω varies from 1.610 to 1.635. The most abundant member of this group occurs as colorless crystalline aggregates and veinlets in pseudowavellite and chert fragments. These aggregates are made up of stubby hexagonal prisms terminated by the unit pyramid; the crystals are rough and generally are subparallel groups rather than single individuals. Their optical properties are: 2V(-)=20°, α =1.622, γ=1.628; negative elongation. The apatite-like minerals are readily recognized as such after some experience. They are generally in recognizable hexagonal forms, and range from colorless to very pale green. They are the only hexagonal minerals found in the nodules. All colorless needle-like crystals or acicular sheaf-like aggregates seen in the nodules by this writer have been shown to be apatite members by x-ray methods. Besides these, all colorless to pale greenish spherules having finely botryoidal surfaces are likewise members of this group. ALUNITE - KAl 3(SO4)2(OH)6 - RhombohedralRound nodules up to eight inches in diameter and angular fragments are made up of about two-thirds alunite and one-third quartz. They are creamy white to dark gray in color, and break with an even conchoiclal fracture; they were originally thought to be chert nodules. They are very even grained, with the grain diameters of both the alunite and quartz averaging about 0.01 mm. The two minerals are uniformly intermixed. Gently crushed fragments in immersion under the microscope show tiny rectangular grains which extinguish parallel to the grain diagonals; they have one index slightly higher than 1.570 and the other somewhat above 1.580. The grains are too small to give interference figures and thus the optical character is not known, but they are believed to be minute cleavage rhombobedra of alunite. The identification was confirmed by its chemical behavior. Heated in a closed tube it gives off acid water. Previous to ignition before the blow pipe it is insoluble in strong acids, but after ignition it is readily soluble in HNO 3. The solution in HNO3 gives a white precipitate when barium chloride is added, indicating SO4; it likewise gives a strong test for sulfur when reduced with charcoal and sodium carbonate. Broken surfaces are readily scratched by a needle, but its hardness appears high for alunite, probably due to the admixture of fine quartz.OTHER MINERALS Quartz is the most abundant mineral of the country rock, comprising strongly brecciated fragments of gray to black chert. Very tiny quartz seams cut the variscite nodules and are associated with pseudowavellite. Tiny quartz crystals coat the surfaces of some of the brecciated chert. On the outside surfaces of some of the variscite nodules, coarse quartz forms rounded milky aggregates. Calcite occurs principally as aggregates of coarse corroded crystals on the outer surfaces of a few variscite nodules; rarely it forms irregular masses on pseudowavellite inside a nodule. Much calcite, apparently original limestone, was seen in thin section surrounding a small nodule made up entirely of pseudowavellite; this calcite was almost opaque to transmitted light because of tiny inclusions of limonite (?). Most of the limonite contains abundant calcite, some in coarse grains. Limonite is abundant as reddish to tan colored earthy material surrounding most of the nodules and commonly filling in between chert fragments. It is in large part incoherent and is mixed with varying amounts of very fine quartz. Some of the lighter colored earthy material may contain clay minerals. Angular black fragments occurring enclosed in phosphate nodules are made up mostly of very fine grained quartz, but also contain evenly distributed tiny grains of an isotropic to weakly birefringent material, ranging in index from 1.58 to 1.62. It is insoluble in boiling HNO 3 both before and after ignition, and thus is probably not a phosphate. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
* Contribution No. 251 from the Department of Mineralogy and Petrography, Harvard University. ** Sterrettite has very recently been shown to he identical with eggonite Bannister, F. A., Mineral. Mag., 26, 131-133 (1941).
|