| Home | AmMin | GMR | RiMG | Collectors Corner | Directory | Short Courses | |
|
|
|||||||

|
|
Volume 48, pages 614-619, 1963
ANTHONYITE AND CALUMETITE, TWO NEW MINERALS FROM THE MICHIGAN COPPER DISTRICT SIDNEY A. WILLIAMS, Department of Geology, Michigan College of Mining and Technology, Houghton, Michigan. ABSTRACT Two new minerals, anthonyite and calumetite, have been found at the Centennial mine near Calumet, Michigan. Both species occur in pockets in, and as encrustations on, basalt. Anthonyite is monoclinic 2/m with a:b:c=.6898:1:.4271 and ß=112°38'; good {100} cleavage. α=1.526, β=1.602, γ=1.602. 2V is 3°, and the mineral is pleochroic with Y and Z a deep smoky blue and X lavender; Z>=Y>X. H=2, sectile; soluble in dilute cold acids. Chemical analysis gave: Cu 41.5, Cl 6.3, AgCl 0.24, OH+H2O+ 35.5, and H2O+ 16.4%. The probable formula is: Cu(OH,Cl)2·3H2O with (OH): (Cl) = 6.36: 1. Calumetite is optically orthorhombic but no single crystals were found. α=1.666, ß=1.690, γ=1.690 with a 2V of 2°. Crystals are feebly pleochroic in blue with Z>=Y>X. H=2, brittle; good {001} cleavage. Chemical analysis gave: Cu 44.1, Cl 5.3, AgCl 0.17, OH+H2O+ 33.5, and H2O+ 16.9%; the probable formula is Cu(OH,Cl)2·2H2O with (OH):(Cl)=8.28:1. Anthonyite is named for John W. Anthony, Associate Professor of Geology, University of Arizona. Calumetite is named for the town near the type locality, Calumet, Michigan. OCCURRENCE Recently Robert H. Dean brought the writer a specimen of a blue mineral from the Centennial mine, Calumet, Michigan which be believed was not azurite, but rather something unusual. Subsequent visits to the mine by Mr. Dean, the writer, and others brought to light a wealth of blue and green copper minerals, few of which were readily identifiable. Subsequent examination indicated that as many as six new species were involved, and this study was then begun. The minerals described here came from various levels in the Centennial mine, and since underground collecting was impossible at the time, the materials were collected by hastily looking through the skips of ore in the headframe, just before they were dumped. The new minerals apparently came from several areas between the 4000- and 5000-levels. Both anthonyite and calumetite were found in cavities and fractures in the basalt wallrock. Associated minerals include capillary tremolite ("byssolite"), quartz, epidote, monazite, and other silicates as well as native copper, cuprite, paratacamite, and numerous unidentified basic copper chlorides. The mode of occurrence of both anthonyite and calumetite suggests that, they are not post-mine evaporites, but that they have formed by the action of chlorine-bearing connate waters on copper, with cuprite as an intermediate step in the alteration.
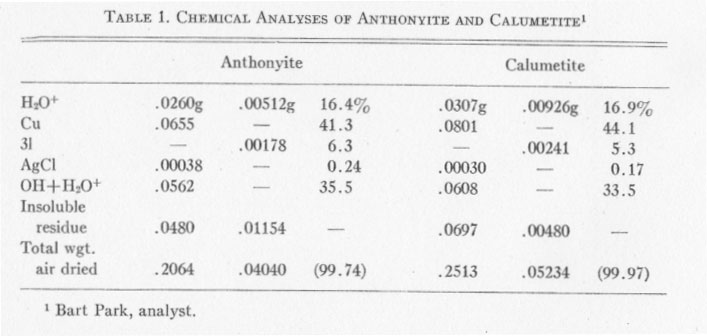
CHEMICAL ANALYSES Chemical analyses of anthonyite and calumetite were performed by Bart Park, Professor of Chemistry, Michigan College of Mining and Technology. Results of the analyses are given in Table 1. Copper was determined electrolytically, silver was determined gravimetrically and calculated to AgCl, and Cl was found gravimetrically as AgCl. Two analyses were run on each mineral; Cu and Ag were determined in the first run but the Cl was lost and had to be determined from a second, smaller sample. The H2O+ was determined by drying to constant weight at 120° C. (for the first sample) and at 140° C. (for the second sample). The values for OH+H2O+ were determined by difference - and the totals were split up into OH and H2O+ on the basis of satisfying valence requirements for cupric copper. Determination of OH+H2O by difference seems justified, since a battery of spectrographic and microchemical tests had indicated that no other elements were present. The insoluble residue consisted largely of quartz with minor amounts of epidote. ANTHONYITE The species anthonyite is named for John W. Anthony, Associate Professor of Geology at the University of Arizona. The formula obtained from the chemical analyses is: Cu.65Cl.18OH1.12·1.82H2O or: Cu(OH,Cl)2·3H2O with the ratio (OH) : (Cl) =6.3:1.
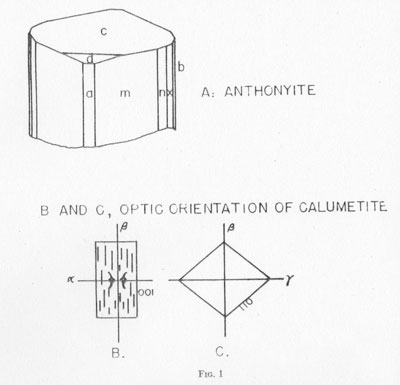
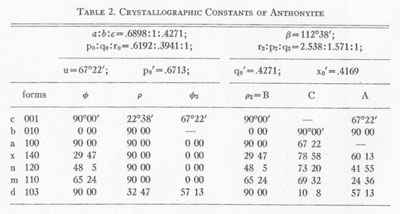
Anthonyite occurs as delicate, lavender prisms up to 0.5 mm in length embedded in and encrusted by an unidentified waxy green basic copper chloride. Other directly associated minerals are copper, cuprite, paratacamite, and an unidentified blue, isotropic, basic copper chloride. Anthonyite is monoclinic 2/m and occurs as crystals which, though small, have a high degree of perfection and are well suited to morphological study. The crystallographic constants and forms are presented in Table 2. An idealized drawing of a crystal is shown in Fig. 1. Many of the crystals are curved one or more times along the c axis; the plane of curvature is {010} and bending apparently occurs readily on the perfect cleavage on {100}.
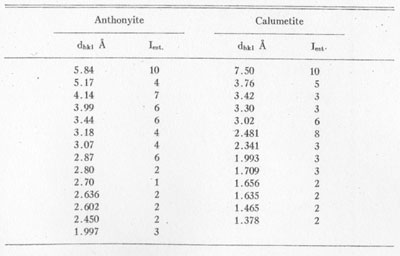
X-ray powder patterns of anthonyite were compared with those of various similar copper minerals and artificial salts1 with no semblance of a match. The strongest lines of anthonyite and calumetite are listed in Table 3.
Few of the gross physical properties could be determined. Anthonyite is quite sectile with crystals bending readily on the {100} cleavage. The hardness is 2. Alteration in air (by dehydration) begins a few weeks after the material has been indoors and then proceeds rapidly. For this reason the specific gravity could not be determined; attempts to perform single crystal x-ray study failed for the same reason.
TABLE 3. STRONGER POWDER PATTERN LINES FOR ANTHONYITE AND
CALUMETITE CoKα RADIATION
Some of the optical properties of anthonyite are enumerated below. The pleochroism is very striking with α=1.526D, Y and Z a deep smoky blue and X a rich lavender; absorption is Z=Y >X. The optic orientation is V=b with the ZΛc angle= +13°. Anthonyite is readily soluble in cold, dilute acids and is insoluble in water. CALUMETITE The species calumetite is named for the town of Calumet, Michigan, near the type locality. The mineral is found in relative abundance at the Centennial mine where it was first discovered, and has since been found at several other nearby mines. Copper minerals closely associated with calumetite are copper, cuprite, malachite, atacamite, paratacamite, buttgenbachite, and two unidentified basic copper chlorides (which are distinct from the two unidentified minerals found with anthonyite). The formula obtained from the analyses of Table 1 is as follows: Cu.69Cl.15OH1.24·1.63H2O or: Cu(OH, Cl)2·2H2O The ratio of (OH) : (Cl) =8.28:1. No single crystals of calumetite were found. It occurs as spherules and sheaves of scaly crystals which are subparallel on the perfect basal cleavage. On the basis of optical data obtained it was concluded that the crystals are orthorhombic with a basal pinacoid {001} and first order prism {110}. The angle between (110) and (110) is about 110°. The optic orientation, shown in Fig. 1, is X=c, Y=a, and Z=b. Other optical data are: α=1.666D The crystals are slightly pleochroic in blue, with absorption Z>Y>X. Calumetite in mass is a brilliant azure blue inclining to powder blue; the basal cleavage shows a pearly luster and is bluish white. The hardness is 2, and the crystals are rather brittle. Measurements of the specific gravity were not reproducible, a fact which reflects on the difficulty of separating the quartz and epidote from the calumetite which coats them. Powder x-ray diffraction data are given in Table 3. Comparison of these lines with those of the compounds listed in the preceding footnote indicates that calumetite is a distinct species. Calumetite is soluble in cold, dilute acids but is insoluble in ammonia and water. Specimens in the writer's possession have lasted for over 8 months with no sign of alteration. ACKNOWLEDGMENTS The writer is indebted to Mr. Robert H. Dean for bringing the problem to his attention. The Calumet and Hecla Corporation, Calumet and Hecla Division, provided funds for the analyses. Messrs. J. P. Pollock and A. W. Schillinger kindly gave the writer permission to collect specimens at the type locality, and showed him every courtesy. Professor Bart Park helped the writer in many fruitful discussions concerning the chemical analyses. Professor John W. Anthony and Dr. A. K. Snelgrove reviewed the manuscript and offered helpful suggestions. NOTE 1 Minerals and salts considered include atacamite, botallackite, paratacamite, and the synthetic salts CuCl2·3Cu(OH)2, CuCl2, CuCl2·2H2O, Cu(OH)Cl, and high-CuCl. Hydromelanathollite and melanotliallite, though ill-defined, do not appear to be similar. Manuscript received, November 9, 1962; accepted for publication January, 11, 1963.
|